Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy
- PMID: 15134625
- PMCID: PMC1871981
- DOI: 10.1215/s1152851703000462
Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy
Abstract
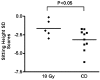
Craniospinal radiation therapy (CSRT) combined with chemotherapy results in significant endocrine morbidity. Between 1987 and 1990, a trial using 18 Gy was conducted to treat 10 young children with medulloblastoma. There were 7 survivors. We compared the endocrine outcome in these children (group 18 Gy) to that of a comparable group treated with conventional doses of CSRT that ranged from 23 to 39 Gy (group CD). Both groups had an identical history of chemotherapy and tumor stage and were treated with recombinant growth hormone therapy (rhGH). The mean age of group 18 Gy at diagnosis was 4.0 years, and rhGH treatment was initiated in 6 children at age 9.2 years. Group CD (12 children) was diagnosed at a mean age of 5.8 years and rhGH started in 11 children at a mean age of 9.6 years. The dose of rhGH used in both groups was identical (0.3 mg/kg/wk). For group 18 Gy, adult heights and sitting heights (a mean standard deviation score of -1.01 +/- 1.11 and -1.62 +/- 1.16, respectively) were statistically greater (P < 0.05) than those for group CD (mean standard deviation score of -2.04 +/- 0.83 and -3.16 +/- 1.43, respectively). Moreover, adult heights of group 18 Gy were not different from midparental heights, unlike group CD, whose adult heights were less than midparental heights (P < 0.0001). Of other endocrine sequelae, 10 patients of the CD group were hypothyroid, 3 had adrenal insufficiency, 3 had hypogonadism, and 2 had early puberty. In contrast, within group 18 Gy, only 1 was hypothyroid (P = 0.006) and 1 had early puberty. We conclude that endocrine morbidity was significantly reduced with 18 Gy CSRT in young children with medulloblastoma.
Figures

Similar articles
-
Adult height and adult sitting height in childhood medulloblastoma survivors.J Clin Endocrinol Metab. 2003 Oct;88(10):4677-81. doi: 10.1210/jc.2003-030619. J Clin Endocrinol Metab. 2003. PMID: 14557440
-
Adult height after growth hormone (GH) treatment for GH deficiency due to cranial irradiation.Med Pediatr Oncol. 2000 Jan;34(1):14-9. doi: 10.1002/(sici)1096-911x(200001)34:1<14::aid-mpo3>3.0.co;2-w. Med Pediatr Oncol. 2000. PMID: 10611579 Clinical Trial.
-
Ovarian function in survivors of childhood medulloblastoma: Impact of reduced dose craniospinal irradiation and high-dose chemotherapy with autologous stem cell rescue.Pediatr Blood Cancer. 2015 Feb;62(2):317-321. doi: 10.1002/pbc.25291. Epub 2014 Oct 24. Pediatr Blood Cancer. 2015. PMID: 25346052
-
Medulloblastoma: clinical and biologic aspects.Neuro Oncol. 1999 Jul;1(3):232-50. doi: 10.1093/neuonc/1.3.232. Neuro Oncol. 1999. PMID: 11550316 Free PMC article. Review.
-
An audit of craniospinal irradiation for medulloblastoma in Newcastle 1970-1992.Clin Oncol (R Coll Radiol). 1995;7(3):179-83. doi: 10.1016/s0936-6555(05)80512-6. Clin Oncol (R Coll Radiol). 1995. PMID: 7547521 Review.
Cited by
-
Survivin, Survivin-2B, and Survivin-deItaEx3 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome.Br J Cancer. 2005 Jan 31;92(2):359-65. doi: 10.1038/sj.bjc.6602317. Br J Cancer. 2005. PMID: 15655550 Free PMC article.
-
Pediatric brain tumor treatment: growth consequences and their management.Pediatr Endocrinol Rev. 2010 Sep;8(1):6-17. Pediatr Endocrinol Rev. 2010. PMID: 21037539 Free PMC article. Review.
-
Production of a SCID mouse model of medulloblastoma to explore the therapeutic value of targeting tumor driver genes.Exp Ther Med. 2021 Feb;21(2):108. doi: 10.3892/etm.2020.9540. Epub 2020 Nov 27. Exp Ther Med. 2021. PMID: 33335571 Free PMC article.
-
Advancing medulloblastoma therapy: strategies and survival insights.Clin Exp Med. 2025 Apr 16;25(1):119. doi: 10.1007/s10238-025-01648-5. Clin Exp Med. 2025. PMID: 40237916 Free PMC article. Review.
-
The Advantage of Proton Therapy in Hypothalamic-Pituitary Axis and Hippocampus Avoidance for Children with Medulloblastoma.Int J Part Ther. 2021 Aug 2;8(3):43-54. doi: 10.14338/IJPT-21-00001.1. eCollection 2022 Winter. Int J Part Ther. 2021. PMID: 35127975 Free PMC article.
References
-
- Adan L, Sainte-Rose C, Souberbielle JC, Zucker JM, Kalifa C, Brauner R. Adult height after growth hormone (GH) treatment for GH deficiency due to cranial irradiation. Med Pediatr Oncol. 2000;34:14–19. - PubMed
-
- Burns EC, Tanner JM, Preece MA, Cameron N. Growth hormone treatment in children with craniopharyngioma: Final growth status. Clin Endocrinol. 1981;14:587–595. - PubMed
-
- CDC/NCHS. Centers for Disease Control, National Center for Health Statistics (2002) 2000 CDC Growth Charts: United States. Available at http://www.cdc.gov/growthcharts - PubMed
-
- Clarson CL, Del Maestro RF. Growth failure after treatment of pediatric brain tumors. Pediatrics. 1999;103:E37. - PubMed
-
- Clayton PE, Shalet SM, Price DA, Addison GM. Growth and growth hormone responses to oxandrolone in boys with constitutional delay of growth and puberty (CDGP) Clin Endocrinol. 1988;29:123–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

