Brain metastases in melanoma: roles of neurotrophins
- PMID: 15134630
- PMCID: PMC1871977
- DOI: 10.1215/s115285170300067x
Brain metastases in melanoma: roles of neurotrophins
Abstract
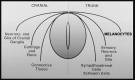
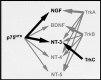
Brain metastasis, which occurs in 20% to 40% of all cancer patients, is an important cause of neoplastic morbidity and mortality. Successful invasion into the brain by tumor cells must include attachment to microvessel endothelial cells, penetration through the blood-brain barrier, and, of relevance, a response to brain survival and growth factors. Neurotrophins (NTs) are important in brain-invasive steps. Human melanoma cell lines express low-affinity NT receptor p75NTR in relation to their brain-metastatic propensity with their invasive properties being regulated by NGF, or nerve growth factor, the prototypic NT. They also express functional TrkC, the putative receptor for the invasion-promoting NT-3. In brain-metastatic melanoma cells, NTs promote invasion by enhancing the production of extracellular matrix (ECM)-degradative enzymes such as heparanase, an enzyme capable of locally destroying both ECM and the basement membrane of the blood-brain barrier. Heparanase is an endo-beta-d-glucuronidase that cleaves heparan sulfate (HS) chains of ECM HS proteoglycans, and it is a unique metastatic determinant because it is the dominant mammalian HS degradative enzyme. Brain-metastatic melanoma cells also produce autocrine/paracrine factors that influence their growth, invasion, and survival in the brain. Synthesis of these factors may serve to regulate NT production by brain cells adjacent to the neoplastic invasion front, such as astrocytes. Increased NT levels have been observed in tumor-adjacent tissues at the invasion front of human brain melanoma. Additionally, astrocytes may contribute to the brain-metastatic specificity of melanoma cells by producing NT-regulated heparanase. Trophic, autocrine, and paracrine growth factors may therefore determine whether metastatic cells can successfully invade, colonize, and grow in the CNS.
Figures


References
-
- Albino AP, Davis BM, Nanus DM. Induction of growth factor RNA expression in human malignant melanoma: Markers of transformation. Cancer Res. 1991;51:4815–4820. - PubMed
-
- Avruch J, Zhang XF, Kyriakis JM. Raf meets Ras: Completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
-
- Barbacid M. Nerve growth factor: A tale of two receptors. Oncogene. 1993;8:2033–2042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

