TorsinA in the nuclear envelope
- PMID: 15136718
- PMCID: PMC419654
- DOI: 10.1073/pnas.0308760101
TorsinA in the nuclear envelope
Abstract
Early-onset torsion dystonia, a CNS-based movement disorder, is usually associated with a single amino acid deletion (Delta E302/303) in the protein torsinA. TorsinA is an AAA+ ATPase in the endoplasmic reticulum, but what it does is unknown. Here, we use torsinA mutants with defects in ATP hydrolysis (E171Q, ATP-bound) and ATP binding (K108A, ATP-free) to probe torsinA's normal cellular function. Surprisingly, ATP-bound torsinA is recruited to the nuclear envelope (NE) of transfected cells, where it alters connections between inner and outer nuclear membranes. In contrast, ATP-free torsinA is diffusely distributed throughout the endoplasmic reticulum and has no effect on the NE. Among AAA+ ATPases, affinity for substrates is high in the ATP-bound and low in the ATP-free state, leading us to propose that component(s) of the NE may be substrates for torsinA. We also find that the disease-promoting Delta E302/303 mutant is in the NE, and that this relocalization, as well as the mutant's previously described ability to induce membranous inclusions, is eliminated by the K108A ATP-binding mutation. These results suggest that changes in interactions involving torsinA in the NE could be important for the pathogenesis of dystonia and point to torsinA and related proteins as a class of ATPases that may operate in the NE.
Figures

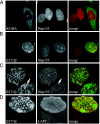
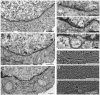
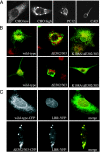
Comment in
-
TorsinA and torsion dystonia: Unraveling the architecture of the nuclear envelope.Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8839-40. doi: 10.1073/pnas.0402441101. Epub 2004 Jun 8. Proc Natl Acad Sci U S A. 2004. PMID: 15187229 Free PMC article. Review. No abstract available.
References
-
- Fahn, S., Bressman, S. B. & Marsden, C. D. (1998) Adv. Neurol. 78, 1-10. - PubMed
-
- Rostasy, K., Augood, S. J., Hewett, J. W., Leung, J. C., Sasaki, H., Ozelius, L. J., Ramesh, V., Standaert, D. G., Breakefield, X. O. & Hedreen, J. C. (2003) Neurobiol. Dis. 12, 11-24. - PubMed
-
- Ghilardi, M. F., Carbon, M., Silvestri, G., Dhawan, V., Tagliati, M., Bressman, S., Ghez, C. & Eidelberg, D. (2003) Ann. Neurol. 54, 102-109. - PubMed
-
- Ozelius, L. J., Hewett, J. W., Page, C. E., Bressman, S. B., Kramer, P. L., Shalish, C., de Leon, D., Brin, M. F., Raymond, D., Corey, D. P., et al. (1997) Nat. Genet. 17, 40-48. - PubMed
-
- Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. (1999) Genome Res. 9, 27-43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

