Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine
- PMID: 15136721
- PMCID: PMC419683
- DOI: 10.1073/pnas.0401418101
Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine
Abstract
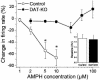
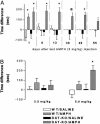
The interaction of amphetamine (AMPH) with the dopamine (DA) transporter (DAT) is thought to be critically important for the DA-elevating actions of this drug. It is commonly believed that DA elevations are involved in the rewarding/reinforcing properties of AMPH and other drugs of abuse. Here, we found that DAT deletion did not eliminate the rewarding effects of AMPH as measured by conditioned place preference (CPP). In fact, mice in which the DAT gene has been deleted (DAT-KO mice) exhibited AMPH-induced CPP for many weeks after the time when extinction occurred in WT mice. Moreover, systemic AMPH still increased extracellular DA in the nucleus accumbens (NAc) of mice lacking the DAT, although local infusion of AMPH into the NAc did not have this effect. By using voltammetry in NAc slices, we found that AMPH did not decrease the rate of DA clearance. The rate of ventral tegmental area DA neuron firing was dramatically inhibited by AMPH in brain slices from WT mice, but there was no inhibition of firing in DAT-KO mice. AMPH-induced CPP was abolished by pretreatment with WAY-100635, a serotonin 5-HT(1A) receptor antagonist, in DAT-KO mice, but the drug did not change AMPH place preference in WT mice. Therefore, despite the absence of the DAT, AMPH displays rewarding effects and causes an increase in extracellular DA in the NAc of DAT-KO mice, acting indirectly in this case. The 5-HT system may be involved in the rewarding effects of AMPH in these mice.
Figures




Similar articles
-
Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter.J Neurosci. 1998 Mar 15;18(6):1979-86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. J Neurosci. 1998. PMID: 9482784 Free PMC article. Review.
-
Neurokinin-1 Antagonism Distinguishes the Role of Norepinephrine Transporter from Dopamine Transporter in Mediating Amphetamine Behaviors.Pharmacology. 2021;106(11-12):597-605. doi: 10.1159/000518033. Epub 2021 Sep 2. Pharmacology. 2021. PMID: 34515205 Free PMC article.
-
Biphasic mechanisms of amphetamine action at the dopamine terminal.J Neurosci. 2014 Apr 16;34(16):5575-82. doi: 10.1523/JNEUROSCI.4050-13.2014. J Neurosci. 2014. PMID: 24741047 Free PMC article.
-
Roles of hippocampal NMDA receptors and nucleus accumbens D1 receptors in the amphetamine-produced conditioned place preference in rats.Brain Res Bull. 2008 Dec 16;77(6):412-9. doi: 10.1016/j.brainresbull.2008.09.007. Epub 2008 Oct 16. Brain Res Bull. 2008. PMID: 18929625
-
Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies.Behav Brain Res. 1999 Jun;101(2):129-52. doi: 10.1016/s0166-4328(99)00022-4. Behav Brain Res. 1999. PMID: 10372570 Review.
Cited by
-
Amphetamine activates an amine-gated chloride channel to generate behavioral effects in Caenorhabditis elegans.J Biol Chem. 2013 Jul 26;288(30):21630-7. doi: 10.1074/jbc.M113.484139. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775081 Free PMC article.
-
Amphetamine action at the cocaine- and antidepressant-sensitive serotonin transporter is modulated by αCaMKII.J Neurosci. 2015 May 27;35(21):8258-71. doi: 10.1523/JNEUROSCI.4034-14.2015. J Neurosci. 2015. PMID: 26019340 Free PMC article.
-
Advancing addiction treatment: what can we learn from animal studies?ILAR J. 2012;53(1):4-13. doi: 10.1093/ilar.53.1.4. ILAR J. 2012. PMID: 23520595 Free PMC article. Review.
-
Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats.J Neurosci. 2018 Feb 21;38(8):1959-1972. doi: 10.1523/JNEUROSCI.1931-17.2018. Epub 2018 Jan 18. J Neurosci. 2018. PMID: 29348190 Free PMC article.
-
Serotonergic involvement in the amelioration of behavioral abnormalities in dopamine transporter knockout mice by nicotine.Neuropharmacology. 2013 Jan;64:348-56. doi: 10.1016/j.neuropharm.2012.07.016. Epub 2012 Jul 15. Neuropharmacology. 2013. PMID: 22809709 Free PMC article.
References
-
- Budygin, E. A., Kilpatrick, M. R., Gainetdinov, R. R. & Wightman, R. M. (2000) Neurosci. Lett. 281, 9-12. - PubMed
-
- Sabeti, J., Gerhardt, G. A. & Zahniser, N. R. (2002) J. Pharmacol. Exp. Ther. 302, 1201-1211. - PubMed
-
- Garris, P. A., Budygin, E. A., Phillips, P. E. M., Venton, B. J., Robinson, D. L., Bergstrom, B. P., Rebec, G. V. & Wightman, R. M. (2003) Neuroscience 118, 819-829. - PubMed
-
- Heikkila, R. E., Orlansky, H. & Cohen, G. (1975) Biochem. Pharmacol. 24, 847-852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

