Cdx2 is essential for axial elongation in mouse development
- PMID: 15136723
- PMCID: PMC419659
- DOI: 10.1073/pnas.0401654101
Cdx2 is essential for axial elongation in mouse development
Abstract
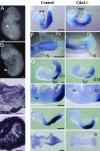
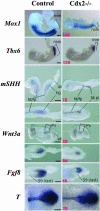
Inactivation of Cdx2 leads to preimplantation embryonic lethality. Rescue of the implantation defect by tetraploid fusion established that Cdx2 is necessary for trophoblastic development, vasculogenesis in the yolk sac mesoderm, allantoic growth, and chorioallantoic fusion. "Rescued" Cdx2 mutants die at late gastrulation stages because of failure of placental development. Cdx2 is also needed for the completion of the normal process of gastrulation and tail bud elongation. Presegmental paraxial mesoderm is severely restricted in amount and somites posterior to somite 5 are abnormal. The Cdx2 mutation, like mutations impairing Wnt and Fgf signaling, causes posterior truncations and disturbs axial patterning of the embryonic structures, indicated by changes in the Hox expression domains. The gene appears to be important in the integration of the pathways controlling embryonic axial elongation, and anterior-posterior patterning.
Figures

References
-
- Moreno, E. & Morata, G. (1999) Nature 400, 873-877. - PubMed
-
- Duprey, P., Chowdhury, K., Dressler, G. R., Balling, R., Simon, D., Guenet, J. L. & Gruss, P. (1988) Genes Dev. 2, 1647-1654. - PubMed
-
- Gamer, L. W. & Wright, C. V. (1993) Mech. Dev. 43, 71-81. - PubMed
-
- James, R. & Kazenwadel, J. (1991) J. Biol. Chem. 266, 3246-3251. - PubMed
-
- Beck, F., Erler, T., Russell, A. & James, R. (1995) Dev. Dyn. 204, 219-227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

