Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene
- PMID: 15136966
- PMCID: PMC1181993
- DOI: 10.1086/422103
Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene
Abstract
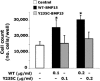
Hypergonadotropic ovarian failure is a common cause of female infertility. It is a heterogeneous disorder that, in the most severe forms, is a result of ovarian dysgenesis (OD). Most OD cases are associated with major X-chromosome abnormalities, but the pathogenesis of this disorder is still largely undefined in patients with a normal karyotype. Animal models showed the important role in female reproduction played by the product of a gene located at Xp11.2 in humans (BMP15). BMP15 is an oocyte-specific growth/differentiation factor that stimulates folliculogenesis and granulosa cell (GC) growth. We report two sisters with a normal karyotype who are affected with hypergonadotropic ovarian failure due to OD. The familial presentation suggested a genetic origin, and candidate genes were screened for mutations. A heterozygous nonconservative substitution in the pro region of BMP15 (Y235C) was identified in both sisters but not in 210 control alleles. This mutation was inherited from the father. Mutant BMP15 appears to be processed abnormally, is associated with reduced GC growth, and antagonizes the stimulatory activity of wild-type protein on GC proliferation. In conclusion, the first natural mutation in human BMP15 is associated with familial OD, indicating that the action of BMP15 is required for the progression of human folliculogenesis. This condition represents an exceptional example of X-linked human disease exclusively affecting heterozygous females who inherited the genetic alteration from the unaffected father. BMP15 defects are involved in the pathogenesis of hypergonadotropic ovarian failure in humans.
Figures




References
Electronic-Database Information
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human BMP15 genomic sequence [accession number AF082349] and human GDF9 mRNA sequence [accession number NM_005260])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
-
- Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R, Hovata O, Dale L, Ritvos O (1999) Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab 84:2744–275010.1210/jc.84.8.2744 - DOI - PubMed
-
- Aittomaki K, Lucena JLD, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de la Chapelle A (1995) Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82:959–968 - PubMed
-
- Brunner AM, Marquardt H, Malacko AR, Lioubin MN, Purchio AF (1989) Site directed mutagenesis of cysteine residues in the pro region of the transforming growth factor β1 precursor. J Biol Chem 264:13660–13664 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

