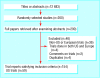
Comparison of reporting of ethnicity in US and European randomised controlled trials
- PMID: 15138158
- PMCID: PMC449871
- DOI: 10.1136/bmj.38061.593935.F7
Comparison of reporting of ethnicity in US and European randomised controlled trials
Similar articles
-
Representation of South Asian people in randomised clinical trials: analysis of trials' data.BMJ. 2003 Jun 7;326(7401):1244-5. doi: 10.1136/bmj.326.7401.1244. BMJ. 2003. PMID: 12791739 Free PMC article. No abstract available.
-
Sex and race/ethnicity reporting in clinical trials: a necessity, not an option.J Womens Health (Larchmt). 2011 Mar;20(3):313-4. doi: 10.1089/jwh.2011.2744. J Womens Health (Larchmt). 2011. PMID: 21351875 No abstract available.
-
Representation of South Asian people in randomised trials: ethnic origin need not be a barrier to participation.BMJ. 2003 Aug 16;327(7411):394-5. doi: 10.1136/bmj.327.7411.394-c. BMJ. 2003. PMID: 12920005 Free PMC article. No abstract available.
-
Quality of reporting of randomized controlled trials of herbal medicine interventions.Am J Med. 2006 Sep;119(9):800.e1-11. doi: 10.1016/j.amjmed.2006.02.006. Am J Med. 2006. PMID: 16945616 Review.
-
The impact of recent European trials on abdominal aortic aneurysm repair: is a paradigm shift warranted?J Surg Res. 2008 Aug;148(2):264-71. doi: 10.1016/j.jss.2007.06.023. Epub 2007 Jul 30. J Surg Res. 2008. PMID: 17996903 Review.
Cited by
-
The performance of the IES-R for Latinos and non-Latinos: Assessing measurement invariance.PLoS One. 2018 Apr 3;13(4):e0195229. doi: 10.1371/journal.pone.0195229. eCollection 2018. PLoS One. 2018. PMID: 29614117 Free PMC article.
-
Why are ethnic minorities under-represented in US research studies?PLoS Med. 2006 Feb;3(2):e49. doi: 10.1371/journal.pmed.0030049. Epub 2005 Dec 27. PLoS Med. 2006. PMID: 16370583 Free PMC article.
-
Progression, symptoms and psychosocial concerns among those severely affected by multiple sclerosis: a mixed-methods cross-sectional study of Black Caribbean and White British people.PLoS One. 2013 Oct 2;8(10):e75431. doi: 10.1371/journal.pone.0075431. eCollection 2013. PLoS One. 2013. PMID: 24098384 Free PMC article.
-
Barriers and facilitators for engaging underrepresented ethnic minority populations in healthcare research: an umbrella review.Int J Equity Health. 2025 Mar 12;24(1):70. doi: 10.1186/s12939-025-02431-4. Int J Equity Health. 2025. PMID: 40075407 Free PMC article.
-
Uneven global and racial representation in major orthopaedic clinical trials: Trends over a decade.J Clin Orthop Trauma. 2022 May 13;29:101894. doi: 10.1016/j.jcot.2022.101894. eCollection 2022 Jun. J Clin Orthop Trauma. 2022. PMID: 35601509 Free PMC article.
References
-
- National Institutes of Health. Monitoring adherence to the NIH policy on the inclusion of women and minorities as subjects in clinical research. Bethesda: NIH, 2001. www4.od.nih.gov/orwh/salmonrpt.pdf (accessed 15 Mar 2004).
-
- Mulrow CD, Oxman AD, eds. Cochrane Collaboration handbook. In: Cochrane Library. Issue 4. Oxford: Update Software, 1997.
-
- Office of Management and Budget. Recommendations from the interagency committee for the review of the racial and ethnic standards to the Office of Management and Budget concerning changes to the standards for the classification of federal data on race and ethnicity. Washington, DC: OoMaB, 1997. (Directive number 15.) www.whitehouse.gov/omb/fedreg/directive_15.html (accessed 15 Mar 2004).
-
- Corbie-Smith G, St George DMM, Moody-Ayers S, Ransohoff DF. Adequacy of reporting race/ethnicity in clinical trials in areas of health disparities. J Clin Epidemiol 2003;56: 416-20. - PubMed
-
- Revised consort statement. www.consort-statement.org (accessed 15 Mar 2004).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources