Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis
- PMID: 15138217
- PMCID: PMC1774060
- DOI: 10.1136/gut.2003.024893
Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis
Abstract
Background and aims: The liver is a major site for the synthesis and actions of platelet activating factor (PAF), a potent hepatic vasoconstrictor and systemic vasodilator. As PAF is implicated in portal hypertension and hyperdynamic circulation associated with liver cirrhosis, we characterised changes in the hepatic PAF system in experimental cirrhosis.
Methods: In rats made cirrhotic by carbon tetrachloride (CCl(4)) administration for eight weeks, we determined hepatic levels of PAF and its cognate receptor, and the effects of PAF and PAF antagonist (BN52021) on portal and arterial pressure.
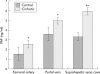
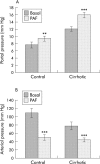
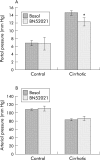
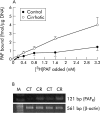
Results: Compared with control rats, cirrhotic rats had higher hepatic PAF levels, higher apparent hepatic efflux of PAF, and higher PAF levels in arterial blood (p<0.01, p<0.01, p<0.05, respectively). Relative to controls, cirrhotic livers had elevated hepatic PAF receptors (by mRNA and protein levels and [(3)H]PAF binding), higher (p<0.01) baseline hepatic portal pressure, and an augmented (p = 0.03) portal pressure response to PAF infusion (1 microg/kg). Portal infusion of BN52021 (5 mg/kg) showed that elevated endogenous PAF was responsible for 23% of the cirrhotic portal pressure increase but made no contribution to systemic hypotension. Finally, increased PAF receptor density was observed in the contractile perisinusoidal stellate cells isolated from cirrhotic livers relative to those from control livers.
Conclusions: In cirrhosis, increased hepatic release of PAF elevates systemic PAF; in combination with upregulated hepatic PAF receptors in stellate cells, this contributes to portal hypertension.
Figures


Similar articles
-
[Influence of platelet activating factor and its antagonist on portal hypertension associated with liver cirrhosis: an experiment with rats].Zhonghua Yi Xue Za Zhi. 2005 Dec 14;85(47):3337-41. Zhonghua Yi Xue Za Zhi. 2005. PMID: 16409839 Chinese.
-
Effect of increased hepatic platelet activating factor and its receptor portal hypertension in CCl4-induced liver cirrhosis.World J Gastroenterol. 2006 Feb 7;12(5):709-15. doi: 10.3748/wjg.v12.i5.709. World J Gastroenterol. 2006. PMID: 16521183 Free PMC article.
-
Hepatic stellate cells may be potential effectors of platelet activating factor induced portal hypertension.World J Gastroenterol. 2008 Jan 14;14(2):218-23. doi: 10.3748/wjg.14.218. World J Gastroenterol. 2008. PMID: 18186558 Free PMC article.
-
Kupffer cells are a major source of increased platelet activating factor in the CCl4-induced cirrhotic rat liver.J Hepatol. 2003 Aug;39(2):200-7. doi: 10.1016/s0168-8278(03)00229-0. J Hepatol. 2003. PMID: 12873816
-
Synthesis of platelet-activating factor and its receptor expression in Kupffer cells in rat carbon tetrachloride-induced cirrhosis.World J Gastroenterol. 2008 Feb 7;14(5):764-70. doi: 10.3748/wjg.14.764. World J Gastroenterol. 2008. PMID: 18205269 Free PMC article.
Cited by
-
Identification of aberrant forms of alkaline sphingomyelinase (NPP7) associated with human liver tumorigenesis.Br J Cancer. 2007 Nov 19;97(10):1441-8. doi: 10.1038/sj.bjc.6604013. Epub 2007 Oct 9. Br J Cancer. 2007. PMID: 17923876 Free PMC article.
-
Platelet activating factor (PAF) antagonism with ginkgolide B protects the liver against acute injury. importance of controlling the receptor of PAF.Dig Dis Sci. 2008 Apr;53(4):1054-62. doi: 10.1007/s10620-007-9982-2. Epub 2007 Oct 13. Dig Dis Sci. 2008. PMID: 17934819
-
Platelet-activating factor in liver injury: a relational scope.World J Gastroenterol. 2006 Jun 21;12(23):3695-706. doi: 10.3748/wjg.v12.i23.3695. World J Gastroenterol. 2006. PMID: 16773686 Free PMC article. Review.
-
Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver.Physiol Rev. 2008 Jan;88(1):125-72. doi: 10.1152/physrev.00013.2007. Physiol Rev. 2008. PMID: 18195085 Free PMC article. Review.
-
Ginkgolide B increases hydrogen sulfide and protects against endothelial dysfunction in diabetic rats.Croat Med J. 2015 Feb;56(1):4-13. doi: 10.3325/cmj.2015.56.4. Croat Med J. 2015. PMID: 25727037 Free PMC article.
References
-
- Snyder F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol 1990;259:C697–708. - PubMed
-
- Snyder F. Platelet-activating factor and its analogs: metabolic pathways and related intracellular processes. Biochim Biophys Acta 1995;1254:231–49. - PubMed
-
- Prescott SM, Zimmerman GA, Stafforini DM, et al. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 2000;69:419–45. - PubMed
-
- Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev 2000;80:1669–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
