Regulation of cell migration and survival by focal adhesion targeting of Lasp-1
- PMID: 15138294
- PMCID: PMC2172195
- DOI: 10.1083/jcb.200311045
Regulation of cell migration and survival by focal adhesion targeting of Lasp-1
Abstract
Large-scale proteomic and functional analysis of isolated pseudopodia revealed the Lim, actin, and SH3 domain protein (Lasp-1) as a novel protein necessary for cell migration, but not adhesion to, the extracellular matrix (ECM). Lasp-1 is a ubiquitously expressed actin-binding protein with a unique domain configuration containing SH3 and LIM domains, and is overexpressed in 8-12% of human breast cancers. We find that stimulation of nonmotile and quiescent cells with growth factors or ECM proteins facilitates Lasp-1 relocalization from the cell periphery to the leading edge of the pseudopodium, where it associates with nascent focal complexes and areas of actin polymerization. Interestingly, although Lasp-1 dynamics in migratory cells occur independently of c-Abl kinase activity and tyrosine phosphorylation, c-Abl activation by apoptotic agents specifically promotes phosphorylation of Lasp-1 at tyrosine 171, which is associated with the loss of Lasp-1 localization to focal adhesions and induction of cell death. Thus, Lasp-1 is a dynamic focal adhesion protein necessary for cell migration and survival in response to growth factors and ECM proteins.
Copyright the Rockefeller University Press
Figures
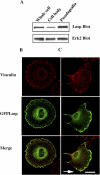
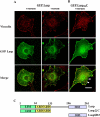
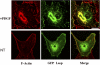



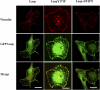
Similar articles
-
Actin binding of human LIM and SH3 protein is regulated by cGMP- and cAMP-dependent protein kinase phosphorylation on serine 146.J Biol Chem. 2003 May 2;278(18):15601-7. doi: 10.1074/jbc.M209009200. Epub 2003 Feb 5. J Biol Chem. 2003. PMID: 12571245
-
Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1.J Biol Chem. 2004 May 7;279(19):20401-10. doi: 10.1074/jbc.M310304200. Epub 2004 Mar 5. J Biol Chem. 2004. PMID: 15004028
-
Lasp-1, a novel type of actin-binding protein accumulating in cell membrane extensions.Mol Med. 1998 Oct;4(10):675-87. Mol Med. 1998. PMID: 9848085 Free PMC article.
-
Focal adhesion regulation of cell behavior.Biochim Biophys Acta. 2004 Jul 5;1692(2-3):103-19. doi: 10.1016/j.bbamcr.2004.04.007. Biochim Biophys Acta. 2004. PMID: 15246682 Review.
-
Focal adhesion kinase: the first ten years.J Cell Sci. 2003 Apr 15;116(Pt 8):1409-16. doi: 10.1242/jcs.00373. J Cell Sci. 2003. PMID: 12640026 Review.
Cited by
-
Loss of the 14-3-3σ is essential for LASP1-mediated colorectal cancer progression via activating PI3K/AKT signaling pathway.Sci Rep. 2016 May 9;6:25631. doi: 10.1038/srep25631. Sci Rep. 2016. PMID: 27156963 Free PMC article.
-
Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein.Int J Ophthalmol. 2018 Aug 18;11(8):1258-1268. doi: 10.18240/ijo.2018.08.03. eCollection 2018. Int J Ophthalmol. 2018. PMID: 30140627 Free PMC article.
-
Comparative transcriptomic analysis reveals gene regulation mediated by caspase activity in a chordate organism.BMC Mol Cell Biol. 2021 Oct 6;22(1):51. doi: 10.1186/s12860-021-00388-0. BMC Mol Cell Biol. 2021. PMID: 34615460 Free PMC article.
-
Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1.Molecules. 2023 Jan 13;28(2):805. doi: 10.3390/molecules28020805. Molecules. 2023. PMID: 36677861 Free PMC article.
-
Nuclear localization and cytosolic overexpression of LASP-1 correlates with tumor size and nodal-positivity of human breast carcinoma.BMC Cancer. 2007 Oct 23;7:198. doi: 10.1186/1471-2407-7-198. BMC Cancer. 2007. PMID: 17956604 Free PMC article.
References
-
- Agami, R., G. Blandino, M. Oren, and Y. Shaul. 1999. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature. 399:809–813. - PubMed
-
- Aizawa, H., Y. Fukui, and I. Yahara. 1997. Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles. J. Cell Sci. 110:2333–2344. - PubMed
-
- Bailly, M., L. Yan, G.M. Whitesides, J.S. Condeelis, and J.E. Segall. 1998. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp. Cell Res. 241:285–299. - PubMed
-
- Bailly, M., I. Ichetovkin, W. Grant, N. Zebda, L.M. Machesky, J.E. Segall, and J. Condeelis. 2001. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr. Biol. 11:620–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

