Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172
- PMID: 15138300
- PMCID: PMC423268
- DOI: 10.1073/pnas.0400997101
Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172
Abstract
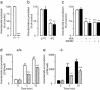
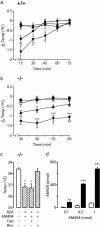
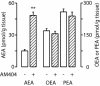
The endogenous cannabinoid anandamide is removed from the synaptic space by a high-affinity transport system present in neurons and astrocytes, which is inhibited by N-(4-hydroxyphenyl)-arachidonamide (AM404). After internalization, anandamide is hydrolyzed by fatty-acid amide hydrolase (FAAH), an intracellular membrane-bound enzyme that also cleaves AM404. Based on kinetic evidence, it has recently been suggested that anandamide internalization may be mediated by passive diffusion driven by FAAH activity. To test this possibility, in the present study, we have investigated anandamide internalization in wild-type and FAAH-deficient (FAAH(-/-)) mice. Cortical neurons from either mouse strain internalized [(3)H]anandamide through a similar mechanism, i.e., via a rapid temperature-sensitive and saturable process, which was blocked by AM404. Moreover, systemic administration of AM404 to either wild-type or FAAH(-/-) mice enhanced the hypothermic effects of exogenous anandamide, a response that was prevented by the CB(1) cannabinoid antagonist rimonabant (SR141716A). The results indicate that anandamide internalization in mouse brain neurons is independent of FAAH activity. In further support of this conclusion, the compound N-(5Z, 8Z, 11Z, 14Z eicosatetraenyl)-4-hydroxybenzamide (AM1172) blocked [(3)H]anandamide internalization in rodent cortical neurons and human astrocytoma cells without acting as a FAAH substrate or inhibitor. AM1172 may serve as a prototype for novel anandamide transport inhibitors with increased metabolic stability.
Figures


Comment in
-
Endocannabinoids: getting the message across.Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8512-3. doi: 10.1073/pnas.0402935101. Epub 2004 Jun 1. Proc Natl Acad Sci U S A. 2004. PMID: 15173576 Free PMC article. Review. No abstract available.
References
-
- Freund, T. F., Katona, I. & Piomelli, D. (2003) Physiol. Rev. 83, 1017–1066. - PubMed
-
- Beltramo, M., Stella, N., Calignano, A., Lin, S. Y., Makriyannis, A. & Piomelli, D. (1997) Science 277, 1094–1097. - PubMed
-
- Hillard, C. J., Edgemond, W. S., Jarrahian, A. & Campbell, W. B. (1997) J. Neurochem. 69, 631–638. - PubMed
-
- Masson, J., Sagne, C., Hamon, M. & El Mestikawy, S. (1999) Pharmacol. Rev. 51, 439–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

