Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle
- PMID: 15138500
- PMCID: PMC406392
- DOI: 10.1371/journal.pbio.0020130
Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle
Abstract
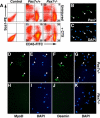
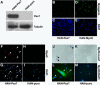
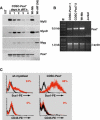
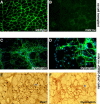
CD45(+):Sca1(+) adult stem cells isolated from uninjured muscle do not display any myogenic potential, whereas those isolated from regenerating muscle give rise to myoblasts expressing the paired-box transcription factor Pax7 and the bHLH factors Myf5 and MyoD. By contrast, CD45(+):Sca1(+) isolated from injured Pax7( -/-) muscle were incapable of forming myoblasts. Infection of CD45(+):Sca1(+) cells from uninjured muscle with retrovirus expressing Pax7 efficiently activated the myogenic program. The resulting myoblasts expressed Myf5 and MyoD and differentiated into myotubes that expressed myogenin and myosin heavy chain. Infection of CD45(-):Sca1(-) cells from Pax7( -/-) muscle similarly gave rise to myoblasts. Notably, infection of Pax7-deficient muscle with adenoviral Pax7 resulted in the de novo formation of regenerated myofibers. Taken together, these results indicate that Pax7 is necessary and sufficient to induce the myogenic specification of CD45(+) stem cells resident in adult skeletal muscle. Moreover, these experiments suggest that viral transduction of Pax7 is a potential therapeutic approach for the treatment of neuromuscular degenerative diseases.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


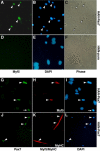
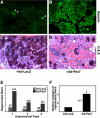
References
-
- Bendall AJ, Ding J, Hu G, Shen MM, Abate-Shen C. Msx1 antagonizes the myogenic activity of Pax3 in migrating limb muscle precursors. Development. 1999;126:4965–4976. - PubMed
-
- Bischoff R. New York: McGraw-Hill; 1994. The satellite cell and muscle regeneration. In: Myogenesis, AG Engel, C Franszini-Armstrong, editors; pp. 97–118.
-
- Blaveri K, Heslop L, Yu DS, Rosenblatt JD, Gross JG, et al. Patterns of repair of dystrophic mouse muscle: Studies on isolated fibers. Dev Dyn. 1999;216:244–256. - PubMed
-
- Borycki AG, Li J, Jin F, Emerson CP, Epstein JA. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–1674. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

