Many ribosomal protein genes are cancer genes in zebrafish
- PMID: 15138505
- PMCID: PMC406397
- DOI: 10.1371/journal.pbio.0020139
Many ribosomal protein genes are cancer genes in zebrafish
Abstract
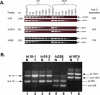
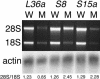
We have generated several hundred lines of zebrafish (Danio rerio), each heterozygous for a recessive embryonic lethal mutation. Since many tumor suppressor genes are recessive lethals, we screened our colony for lines that display early mortality and/or gross evidence of tumors. We identified 12 lines with elevated cancer incidence. Fish from these lines develop malignant peripheral nerve sheath tumors, and in some cases also other tumor types, with moderate to very high frequencies. Surprisingly, 11 of the 12 lines were each heterozygous for a mutation in a different ribosomal protein (RP) gene, while one line was heterozygous for a mutation in a zebrafish paralog of the human and mouse tumor suppressor gene, neurofibromatosis type 2. Our findings suggest that many RP genes may act as haploinsufficient tumor suppressors in fish. Many RP genes might also be cancer genes in humans, where their role in tumorigenesis could easily have escaped detection up to now.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

