The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA
- PMID: 15139850
- PMCID: PMC1133946
- DOI: 10.1042/BJ20040469
The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA
Abstract
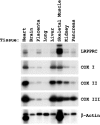
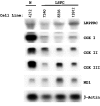
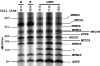
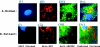
Leigh syndrome French Canadian (LSFC) is a variant of cytochrome oxidase deficiency found in Québec and caused by mutations in the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene. Northern blots showed that the LRPPRC mRNA levels seen in skeletal muscle>heart>placenta>kidney>liver>lung=brain were proportionally almost opposite in strength to the severity of the enzymic cytochrome oxidase defect. The levels of COX (cytochrome c oxidase) I and COX III mRNA visible on Northern blots were reduced in LSFC patients due to the common (A354V, Ala354-->Val) founder mutation. The amount of LRPPRC protein found in both fibroblast and liver mitochondria from LSFC patients was consistently reduced to <30% of control levels. Import of [(35)S]methionine LRPPRC into rat liver mitochondria was slower for the mutant (A354V) protein. A titre of LRPPRC protein was also found in nuclear fractions that could not be easily accounted for by mitochondrial contamination. [35S]Methionine labelling of mitochondrial translation products showed that the translation of COX I, and perhaps COX III, was specifically reduced in the presence of the mutation. These results suggest that the gene product of LRPPRC, like PET 309p, has a role in the translation or stability of the mRNA for mitochondrially encoded COX subunits. A more diffuse distribution of LRPPRC in LSFC cells compared with controls was evident when viewed by immunofluorescence microscopy, with less LRPPRC present in peripheral mitochondria.
Figures


References
-
- Manthey G. M., Przybyla-Zawislak B. D., McEwen J. E. The Saccharomyces cerevisiae Pet309 protein is embedded in the mitochondrial inner membrane. Eur. J. Biochem. 1998;255:156–161. - PubMed
-
- Hou J., Wang F., McKeehan W. L. Molecular cloning and expression of the gene for a major leucine-rich protein from human hepatoblastoma cells (HepG2) In Vitro Cell. Dev. Biol. Anim. 1994;30A:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

