A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance
- PMID: 15140400
- PMCID: PMC1502091
- DOI: 10.1593/neo.03205
A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance
Abstract
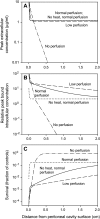
A theoretical model for the intraperitoneal (i.p.) delivery of cisplatin and heat to tumor metastases in tissues adjacent to the peritoneal cavity is presented. The penetration distance (the depth to which drug diffuses directly from the cavity into tissues) is predicted to be on the order of 0.5 mm. The model shows that exchange with the microvasculature has more effect than cellular uptake in limiting the penetration distance. Possible effects of hyperthermia are simulated, including increased cell uptake of drug, increased cell kill at a given level of intracellular drug, and decreased microvascular density. The model suggests that the experimental finding of elevated intracellular platinum levels up to a depth of 3 to 5 mm when drug is delivered i.p. by a heated infusion solution is due to penetration of heat to this distance, causing increased cell uptake of drug. Beyond a depth of about 0.5 mm, the drug is delivered mainly through the circulation. Use of sodium thiosulfate to deactivate systemic cisplatin may therefore be counterproductive when heat is delivered locally. The model suggests that i.p. delivery of heat, combined with systemic delivery of drug, may be as effective as i.p. delivery of heat and drug.
Figures




Similar articles
-
Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines.Eur J Cancer. 1998 Jan;34(1):148-54. doi: 10.1016/s0959-8049(97)00370-5. Eur J Cancer. 1998. PMID: 9624250 Clinical Trial.
-
Population pharmacokinetics of cisplatin in patients with advanced ovarian cancer during intraperitoneal hyperthermia chemotherapy.Anticancer Res. 2002 Mar-Apr;22(2B):1329-36. Anticancer Res. 2002. PMID: 12168946
-
High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis.Ann Surg. 2006 Jul;244(1):106-12. doi: 10.1097/01.sla.0000218089.61635.5f. Ann Surg. 2006. PMID: 16794395 Free PMC article.
-
Intraperitoneal perfusion chemotherapy with hyperthermia in some malignant ascites.Khirurgiia (Sofiia). 2013;(4):11-8. Khirurgiia (Sofiia). 2013. PMID: 24800315 Review. Bulgarian, English.
-
Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: from laboratory bench to bedside.Cancer Treat Rev. 2006 Oct;32(6):471-82. doi: 10.1016/j.ctrv.2006.07.006. Epub 2006 Aug 30. Cancer Treat Rev. 2006. PMID: 16942841 Review.
Cited by
-
Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies.World J Gastroenterol. 2016 Aug 14;22(30):6906-16. doi: 10.3748/wjg.v22.i30.6906. World J Gastroenterol. 2016. PMID: 27570426 Free PMC article. Review.
-
Optimal drug delivery for intraperitoneal paclitaxel (PTX) in murine model.Pleura Peritoneum. 2017 Jun 1;2(2):95-102. doi: 10.1515/pp-2017-0002. Epub 2017 Mar 30. Pleura Peritoneum. 2017. PMID: 30911637 Free PMC article.
-
Mathematical Based Calculation of Drug Penetration Depth in Solid Tumors.Biomed Res Int. 2016;2016:8437247. doi: 10.1155/2016/8437247. Epub 2016 Jun 8. Biomed Res Int. 2016. PMID: 27376087 Free PMC article.
-
Multiscale tumor spatiokinetic model for intraperitoneal therapy.AAPS J. 2014 May;16(3):424-39. doi: 10.1208/s12248-014-9574-y. Epub 2014 Feb 26. AAPS J. 2014. PMID: 24570339 Free PMC article.
-
Modelling drug transport during intraperitoneal chemotherapy.Pleura Peritoneum. 2017 Jun 1;2(2):73-83. doi: 10.1515/pp-2017-0004. Epub 2017 Apr 14. Pleura Peritoneum. 2017. PMID: 30911635 Free PMC article. Review.
References
-
- Akyurekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. Int J Hypertherm. 1997;13:481–496. - PubMed
-
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. - PubMed
-
- Bartlett DL, Buell JF, Libutti SK, Reed E, Lee KB, Figg WD, Venzon DJ, Alexander HR. A phase I trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cisplatin in the treatment of peritoneal carcinomatosis. Cancer. 1998;83:1251–1261. - PubMed
-
- Blakley BW, Cohen JI, Doolittle ND, Muldoon LL, Campbell KC, Dickey DT, Neuwelt DT. Strategies for prevention of toxicity caused by platinum-based chemotherapy: review and summary of the annual meeting of the Blood-Brain Barrier Disruption Program, Gleneden Beach, Oregon, March 10, 2001. Laryngoscope. 2002;112:1997–2001. - PubMed
-
- Ceelen WP, Hesse U, de Hemptinne B, Pattyn P. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg. 2000;87:1006–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
