MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis
- PMID: 15140741
- PMCID: PMC7974468
MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis
Abstract
Background and purpose: Intracranial manifestations of Langerhans cell histiocytosis (LCH) are underestimated in frequency and diversity. We categorized the spectrum of MR imaging changes in LCH.
Methods: We retrospectively reviewed 474 MR images in 163 patients with LCH and 55 control subjects. Lesions were characterized by anatomic region and signal intensity. Brain atrophy was assessed.
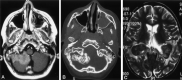
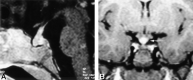
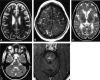
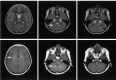
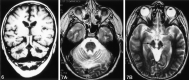
Results: We noted osseous lesions in the craniofacial or skull bones in 56% of patients, meningeal lesions in 29%, and choroid-plexus involvement in 6%. In the hypothalamic-pituitary region, infundibular thickening occurred in 50%; pronounced hypothalamic mass lesions in 10%; and infundibular atrophy in 29%. The pineal gland had a cystic appearance in 28%, and pineal-gland enlargement (>10 mm) was noted in 14%. Nonspecific paranasal-sinus or mastoid opacifications were seen in 55% of patients versus 20% of controls, and accentuated Virchow-Robin spaces occurred in 70% of patients versus 27% of controls (P <.001). Intra-axial, white-matter parenchymal changes resulted in a leukoencephalopathy-like pattern in 36%. Enhancing lesions in a vascular distribution were noted in 5%. Gray-matter changes suggestive of neurodegeneration were identified in the cerebellar dentate nucleus in 40% and in the supratentorial basal ganglia in 26%. All patients with neurodegenerative lesions had lesions in the extra-axial spaces. Cerebral atrophy was found in 8%.
Conclusion: In LCH, cranial and intracranial changes at MR imaging include 1) lesions of the craniofacial bone and skull base with or without soft-tissue extension; 2) intracranial, extra-axial changes (hypothalamic-pituitary region, meninges, circumventricular organs); 3) intracranial, intra-axial changes (white matter and gray matter); and 4) cerebral atrophy.
Figures

References
-
- Arceci RJ. The histiocytoses: the fall of the Tower of Babel. Eur J Cancer 1999;35:747–767 - PubMed
-
- Egeler RM, D’Angio GJ. Langerhans cell histiocytosis. J Pediatr 1995;127:1–11 - PubMed
-
- Grois N, Flucher-Wolfram B, Heitger A, Mostbeck GH, Hofmann J, Gadner H. Diabetes insipidus in Langerhans cell histiocytosis: results from the DAL-HX 83 study. Med Pediatr Oncol 1995;24:248–256 - PubMed
-
- Nanduri VR, Bareille P, Pritchard J, Stanhope R. Growth and endocrine disorders in multisystem Langerhans’ cell histiocytosis. Clin Endocrinol (Oxf) 2000;53:509–515 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
