Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3
- PMID: 15140769
- PMCID: PMC1755017
- DOI: 10.1136/ard.2003.007229
Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3
Abstract
Background: Galectin-3 is a lectin detected in mature and early hypertrophic chondrocytes; osteoarthritic (OA) chondrocytes can re-express hypertrophic markers.
Objective: To investigate the synthesis and subcellular localisation of galectin-3 in adult chondrocytes as well as the possibility of cleavage of galectin-3 by collagenase-1 and -3.
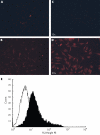
Methods: Galectin-3 was assessed by immunohistochemistry and real time polymerase chain reaction (PCR) in normal and OA cartilage. Its localisation was investigated by subcellular fractionation, immunocytology, and flow cytometry. Proteolysis of galectin-3 by collagenase-1 and -3 was determined by in vitro assay.
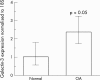
Results: Galectin-3 expression was increased 2.4-fold as measured by reverse transcriptase (RT)-PCR (p<0.05, n = 5) and threefold by immunohistochemistry (p<0.003 n = 6) in OA cartilage compared with normal cartilage. In adult chondrocytes, galectin-3 was found in the cytosol and membrane enriched fractions. Both immunocytology and flow cytometry confirmed the presence of galectin-3 at the surface of chondrocytes. A strong correlation was found between integrin-beta1 and galectin-3 expression at the surface of chondrocytes. Moreover, collagenase-3 cleaved galectin-3 with a higher activity than collagenase-1. The proteolysed sites generated were identical to those produced by gelatinases A and B.
Conclusion: Galectin-3 may play a part in OA, having two roles, one intracellular and not yet identified, and another at the cell surface, possibly related to the interaction of chondrocytes and the cartilage matrix.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

