Local sensory cues and place cell directionality: additional evidence of prospective coding in the hippocampus
- PMID: 15140925
- PMCID: PMC6729394
- DOI: 10.1523/JNEUROSCI.4896-03.2004
Local sensory cues and place cell directionality: additional evidence of prospective coding in the hippocampus
Abstract
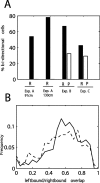
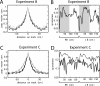
In tasks involving goal-directed, stereotyped trajectories on uniform tracks, the spatially selective activity of hippocampal principal cells depends on the animal's direction of motion. Principal cell ensemble activity while the rat moves in opposite directions through a given location is typically uncorrelated. It is shown here, with data from three experiments, that multimodal, local sensory cues can change the directional properties of CA1 pyramidal cells, inducing bidirectionality in a significant proportion of place cells. For a majority of these bidirectional place cells, place field centers in the two directions of motion were displaced relative to one another, as would be the case if the cells were representing a position in space approximately 5-10 cm ahead of the rat or if place cells were subject to strong accommodation or inhibition in the latter half of their input fields. However, place field density was not affected by the presence of local cues, but in the experimental condition with the most salient sensory cues, the CA1 population vectors in the "cue-rich" condition were sparser and changed more quickly in space than in the "cue-poor" condition. These results suggest that "view-invariant" object representations are projected to the hippocampus from lower cortical areas and can have the effect of increasing the correlation of the hippocampal input vectors in the two directions, hence decreasing the orthogonality of hippocampal output.
Figures





References
-
- Barnes CA, Suster MS, Shen J, McNaughton BL (1997) Multistability of cognitive maps in the hippocampus of old rats. Nature 388: 272-275. - PubMed
-
- Battaglia FP, Sutherland GL, McNaughton BL (2002) Predictive code in bidirectional place fields. Soc Neurosci Abstr 28: 678.12.
-
- Best PJ, White AM, Minai A (2001) Spatial processing in the brain: the activity of hippocampal place cells. Annu Rev Neurosci 24: 459-486. - PubMed
-
- Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193-205. - PubMed
-
- Bower MR (2003) The neural basis of trajectory computations in rodent-posterior-parietal cortex and hippocampus. PhD thesis, University of Arizona.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
