Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance
- PMID: 15140929
- PMCID: PMC2376823
- DOI: 10.1523/JNEUROSCI.5552-03.2004
Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance
Abstract
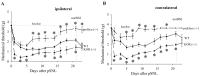
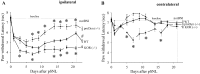
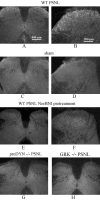
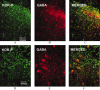
Release of endogenous dynorphin opioids within the spinal cord after partial sciatic nerve ligation (pSNL) is known to contribute to the neuropathic pain processes. Using a phosphoselective antibody [kappa opioid receptor (KOR-P)] able to detect the serine 369 phosphorylated form of the KOR, we determined possible sites of dynorphin action within the spinal cord after pSNL. KOR-P immunoreactivity (IR) was markedly increased in the L4-L5 spinal dorsal horn of wild-type C57BL/6 mice (7-21 d) after lesion, but not in mice pretreated with the KOR antagonist nor-binaltorphimine (norBNI). In addition, knock-out mice lacking prodynorphin, KOR, or G-protein receptor kinase 3 (GRK3) did not show significant increases in KOR-P IR after pSNL. KOR-P IR was colocalized in both GABAergic neurons and GFAP-positive astrocytes in both ipsilateral and contralateral spinal dorsal horn. Consistent with sustained opioid release, KOR knock-out mice developed significantly increased tactile allodynia and thermal hyperalgesia in both the early (first week) and late (third week) interval after lesion. Similarly, mice pretreated with norBNI showed enhanced hyperalgesia and allodynia during the 3 weeks after pSNL. Because sustained activation of opioid receptors might induce tolerance, we measured the antinociceptive effect of the kappa agonist U50,488 using radiant heat applied to the ipsilateral hindpaw, and we found that agonist potency was significantly decreased 7 d after pSNL. In contrast, neither prodynorphin nor GRK3 knock-out mice showed U50,488 tolerance after pSNL. These findings suggest that pSNL induced a sustained release of endogenous prodynorphin-derived opioid peptides that activated an anti-nociceptive KOR system in mouse spinal cord. Thus, endogenous dynorphin had both pronociceptive and antinociceptive actions after nerve injury and induced GRK3-mediated opioid tolerance.
Figures


Similar articles
-
Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase.J Neurosci. 2007 Mar 7;27(10):2570-81. doi: 10.1523/JNEUROSCI.3728-06.2007. J Neurosci. 2007. PMID: 17344394 Free PMC article.
-
Involvement of the dynorphin/KOR system on the nociceptive, emotional and cognitive manifestations of joint pain in mice.Neuropharmacology. 2017 Apr;116:315-327. doi: 10.1016/j.neuropharm.2016.08.026. Epub 2016 Aug 24. Neuropharmacology. 2017. PMID: 27567942
-
Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes.J Biol Chem. 2006 Jun 30;281(26):18081-9. doi: 10.1074/jbc.M513640200. Epub 2006 Apr 28. J Biol Chem. 2006. PMID: 16648139 Free PMC article.
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
-
Molecular Genetics of Kappa Opioids in Pain and Itch Sensations.Handb Exp Pharmacol. 2022;271:255-274. doi: 10.1007/164_2020_397. Handb Exp Pharmacol. 2022. PMID: 33145633 Free PMC article. Review.
Cited by
-
Repeated stress dysregulates κ-opioid receptor signaling in the dorsal raphe through a p38α MAPK-dependent mechanism.J Neurosci. 2012 Sep 5;32(36):12325-36. doi: 10.1523/JNEUROSCI.2053-12.2012. J Neurosci. 2012. PMID: 22956823 Free PMC article.
-
MicroRNA-7a ameliorates neuropathic pain in a rat model of spinal nerve ligation via the neurofilament light polypeptide-dependent signal transducer and activator of transcription signaling pathway.Mol Pain. 2019 Jan-Dec;15:1744806919842464. doi: 10.1177/1744806919842464. Mol Pain. 2019. PMID: 30987515 Free PMC article.
-
Usefulness of knockout mice to clarify the role of the opioid system in chronic pain.Br J Pharmacol. 2018 Jul;175(14):2791-2808. doi: 10.1111/bph.14088. Epub 2018 Jan 6. Br J Pharmacol. 2018. PMID: 29124744 Free PMC article. Review.
-
Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase.J Neurosci. 2007 Mar 7;27(10):2570-81. doi: 10.1523/JNEUROSCI.3728-06.2007. J Neurosci. 2007. PMID: 17344394 Free PMC article.
-
Sex differences in kappa opioid pharmacology.Life Sci. 2011 Jan 3;88(1-2):2-16. doi: 10.1016/j.lfs.2010.10.007. Epub 2010 Oct 14. Life Sci. 2011. PMID: 20951148 Free PMC article. Review.
References
-
- Appleyard SM, Patterson TA, Jin W, Chavkin C (1997) Agonist-induced phosphorylation of the kappa-opioid receptor. J Neuroses 69: 2405-2412. - PubMed
-
- Arner S, Meyerson BA (1988) Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain 33: 11-23. - PubMed
-
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW, Elde R (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA 92: 5062-5066. - PMC - PubMed
-
- Barres BA (1991) Glial ion channels. Curr Opin Neurobiol 1: 354-359. - PubMed
-
- Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
