Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors
- PMID: 15140932
- PMCID: PMC6729402
- DOI: 10.1523/JNEUROSCI.0515-03.2004
Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors
Abstract
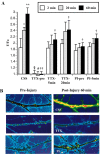
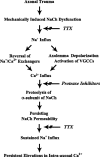
We demonstrated previously that dynamic stretch injury of cultured axons induces structural changes and Ca2+ influx modulated by tetrodotoxin (TTX)-sensitive voltage-gated sodium channels (NaChs). In the present study, we evaluated potential damage to the NaCh alpha-subunit, which can cause noninactivation of NaChs. In addition, we explored the effects of pre-injury and post-injury treatment with TTX and protease inhibition on proteolysis of the NaCh alpha-subunit and intra-axonal calcium levels ([Ca2+]i) over 60 min after trauma. After stretch injury, we found that [Ca2+]i continued to increase in untreated axons for at least 60 min. We also observed that the III-IV intra-axonal loop of the NaCh alpha-subunit was proteolyzed between 5 and 20 min after trauma. Pre-injury treatment of the axons with TTX completely abolished the posttraumatic increase in [Ca2+]i and proteolysis of the NaCh alpha-subunit. In addition, both pre-injury and post-injury inhibition of protease activity attenuated long-term increases in [Ca2+]i as well as mitigating degradation of the NaCh alpha-subunit. These results suggest a unique "feed-forward" deleterious process initiated by mechanical trauma of axons. Na+ influx through NaChs resulting from axonal deformation triggers initial increases in [Ca2+]i and subsequent proteolysis of the NaCh-subunit. In turn, degradation of the alpha-subunit promotes persistent elevations in [Ca2+]i, fueling additional pathologic changes. These observations may have important implications for developing therapeutic strategies for axonal trauma.
Figures



Similar articles
-
Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels.J Neurosci. 2001 Mar 15;21(6):1923-30. doi: 10.1523/JNEUROSCI.21-06-01923.2001. J Neurosci. 2001. PMID: 11245677 Free PMC article.
-
Sodium channelopathy induced by mild axonal trauma worsens outcome after a repeat injury.J Neurosci Res. 2009 Dec;87(16):3620-5. doi: 10.1002/jnr.22161. J Neurosci Res. 2009. PMID: 19565655 Free PMC article.
-
Mechanisms of calpain mediated proteolysis of voltage gated sodium channel α-subunits following in vitro dynamic stretch injury.J Neurochem. 2012 Jun;121(5):793-805. doi: 10.1111/j.1471-4159.2012.07735.x. Epub 2012 Apr 12. J Neurochem. 2012. PMID: 22428606 Free PMC article.
-
Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin.Channels (Austin). 2008 Nov-Dec;2(6):407-12. doi: 10.4161/chan.2.6.7429. Epub 2008 Nov 12. Channels (Austin). 2008. PMID: 19098433 Review.
-
Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon.Neurobiol Dis. 2013 Dec;60:61-79. doi: 10.1016/j.nbd.2013.08.010. Epub 2013 Aug 19. Neurobiol Dis. 2013. PMID: 23969238 Free PMC article. Review.
Cited by
-
Disruption of Network Synchrony and Cognitive Dysfunction After Traumatic Brain Injury.Front Syst Neurosci. 2016 May 17;10:43. doi: 10.3389/fnsys.2016.00043. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27242454 Free PMC article.
-
Modelling human pathology of traumatic brain injury in animal models.J Intern Med. 2019 Jun;285(6):594-607. doi: 10.1111/joim.12909. Epub 2019 Apr 23. J Intern Med. 2019. PMID: 30963638 Free PMC article. Review.
-
A computational study of invariant I5 in a nearly incompressible transversely isotropic model for white matter.J Biomech. 2017 May 24;57:146-151. doi: 10.1016/j.jbiomech.2017.03.025. Epub 2017 Apr 9. J Biomech. 2017. PMID: 28433390 Free PMC article.
-
High Ca2+ Influx During Traumatic Brain Injury Leads to Caspase-1-Dependent Neuroinflammation and Cell Death.Mol Neurobiol. 2017 Aug;54(6):3964-3975. doi: 10.1007/s12035-016-9949-4. Epub 2016 Jun 11. Mol Neurobiol. 2017. PMID: 27289225 Free PMC article.
-
Focal increases of axoplasmic Ca2+, aggregation of sodium-calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress.J Neurosci. 2012 Aug 29;32(35):12028-37. doi: 10.1523/JNEUROSCI.0408-12.2012. J Neurosci. 2012. PMID: 22933787 Free PMC article.
References
-
- Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12: 557-563. - PubMed
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McClellan DR (1989) Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology 15: 49-59. - PubMed
-
- Banik NL, Hogan EL, Hsu CY (1987) The multimolecular cascade of spinal cord injury. Studies on prostanoids, calcium, and proteinases. Neurochem Pathol 7: 57-77. - PubMed
-
- Benz I, Beck W, Kraas W, Stoll D, Jung G, Kohlhardt M (1997) Two types of modified cardiac Na+ channels after cytosolic interventions at the alpha-subunit capable of removing Na+ inactivation. Eur Biophys J 25: 189-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
