Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy
- PMID: 15140937
- PMCID: PMC6729383
- DOI: 10.1523/JNEUROSCI.0797-04.2004
Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy
Abstract
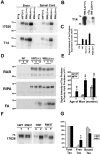
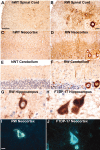
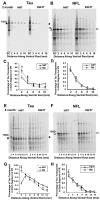
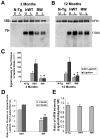
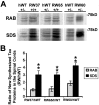
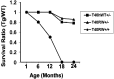
Intracellular accumulations of filamentous tau inclusions are neuropathological hallmarks of neurodegenerative diseases known as tauopathies. The discovery of multiple pathogenic tau gene mutations in many kindreds with familial frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) unequivocally confirmed the central role of tau abnormalities in the etiology of neurodegenerative disorders. To examine the effects of tau gene mutations and the role of tau abnormalities in neurodegenerative tauopathies, transgenic (Tg) mice were engineered to express the longest human tau isoform (T40) with or without the R406W mutation (RW and hWT Tg mice, respectively) that is pathogenic for FTDP-17 in several kindreds. RW but not hWT tau Tg mice developed an age-dependent accumulation of insoluble filamentous tau aggregates in neuronal perikarya of the cerebral cortex, hippocampus, cerebellum, and spinal cord. Significantly, CNS axons in RW mice contained reduced levels of tau when compared with hWT mice, and this was linked to retarded axonal transport and increased accumulation of an insoluble pool of RW but not hWT tau. Furthermore, RW but not hWT mice demonstrated neurodegeneration and a reduced lifespan. These data indicate that the R406W mutation causes reduced binding of this mutant tau to microtubules, resulting in slower axonal transport. This altered tau function caused by the RW mutation leads to increased accumulation and reduced solubility of RW tau in an age-dependent manner, culminating in the formation of filamentous intraneuronal tau aggregates similar to that observed in tauopathy patients.
Figures


References
-
- Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow E, Mandelkow E (2000) Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 39: 11714-11721. - PubMed
-
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo MG, Spillantini F, Tagliavini M, Goedert M, Ghetti B (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 58: 667-677. - PubMed
-
- Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D'Souza I, Lee VM-Y, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC (1998) Pathogenic implications of mutations in the tau gene in pallidopontonigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA 95: 13103-13107. - PMC - PubMed
-
- Connell JW, Gibb GM, Betts JC, Blackstock JC, Gallo J, Lovestone S, Hutton M, Anderton BH (2001) Effects of FTDP-17 mutations on the in vitro phosphorylation of tau by glycogen synthase kinase 3β identified by mass spectrometry demonstrate certain mutations exert long-range conformational changes. FEBS Lett 493: 40-44. - PubMed
-
- Crowther RA, Goedert M (2000) Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol 130: 271-279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
