Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2
- PMID: 15140939
- PMCID: PMC6729393
- DOI: 10.1523/JNEUROSCI.5265-03.2004
Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2
Abstract
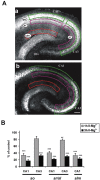
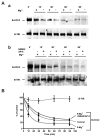
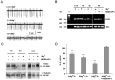
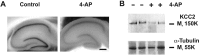
GABA-mediated fast-hyperpolarizing inhibition depends on extrusion of chloride by the neuron-specific K-Cl cotransporter, KCC2. Here we show that sustained interictal-like activity in hippocampal slices downregulates KCC2 mRNA and protein expression in CA1 pyramidal neurons, which leads to a reduced capacity for neuronal Cl- extrusion. This effect is mediated by endogenous BDNF acting on tyrosine receptor kinase B (TrkB), with down-stream cascades involving both Shc/FRS-2 (src homology 2 domain containing transforming protein/FGF receptor substrate 2) and PLCgamma (phospholipase Cgamma)-cAMP response element-binding protein signaling. The plasmalemmal KCC2 has a very high rate of turnover, with a time frame that suggests a novel role for changes in KCC2 expression in diverse manifestations of neuronal plasticity. A downregulation of KCC2 may be a general early response involved in various kinds of neuronal trauma.
Figures


Similar articles
-
BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion.J Cell Biol. 2002 Dec 9;159(5):747-52. doi: 10.1083/jcb.200209011. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473684 Free PMC article.
-
Mechanism of TrkB-mediated hippocampal long-term potentiation.Neuron. 2002 Sep 26;36(1):121-37. doi: 10.1016/s0896-6273(02)00942-x. Neuron. 2002. PMID: 12367511
-
Stimulation of neuropeptide Y gene expression by brain-derived neurotrophic factor requires both the phospholipase Cgamma and Shc binding sites on its receptor, TrkB.Biochem J. 1998 Aug 1;333 ( Pt 3)(Pt 3):505-9. doi: 10.1042/bj3330505. Biochem J. 1998. PMID: 9677306 Free PMC article.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2.Trends Neurosci. 2013 Dec;36(12):726-737. doi: 10.1016/j.tins.2013.08.006. Epub 2013 Oct 15. Trends Neurosci. 2013. PMID: 24139641 Free PMC article. Review.
Cited by
-
Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters.Front Cell Neurosci. 2015 May 12;9:173. doi: 10.3389/fncel.2015.00173. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26029047 Free PMC article.
-
Dose-dependent reversal of KCC2 hypofunction and phenobarbital-resistant neonatal seizures by ANA12.Sci Rep. 2018 Aug 10;8(1):11987. doi: 10.1038/s41598-018-30486-7. Sci Rep. 2018. PMID: 30097625 Free PMC article.
-
Time-matched pre- and postsynaptic changes of GABAergic synaptic transmission in the developing mouse superior colliculus.J Physiol. 2005 Mar 15;563(Pt 3):795-807. doi: 10.1113/jphysiol.2004.081216. Epub 2005 Jan 20. J Physiol. 2005. PMID: 15661815 Free PMC article.
-
Maternal taurine as a modulator of Cl- homeostasis as well as of glycine/GABAA receptors for neocortical development.Front Cell Neurosci. 2023 Aug 3;17:1221441. doi: 10.3389/fncel.2023.1221441. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37601283 Free PMC article. Review.
-
Striatal Chloride Dysregulation and Impaired GABAergic Signaling Due to Cation-Chloride Cotransporter Dysfunction in Huntington's Disease.Front Cell Neurosci. 2022 Jan 14;15:817013. doi: 10.3389/fncel.2021.817013. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35095429 Free PMC article. Review.
References
-
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E (2003) BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development 130: 1267-1280. - PubMed
-
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA (1986) Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res 398: 215-219. - PubMed
-
- Avoli M (1996) GABA-mediated synchronous potentials and seizure generation. Epilepsia 37: 1035-1042. - PubMed
-
- Avoli M, Louvel J, Drapeau C, Pumain R, Kurcewicz I (1995) GABAA-mediated inhibition and in vitro epileptogenesis in the human neocortex. J Neurophysiol 73: 468-484. - PubMed
-
- Beau FE, Alger BE (1998) Transient suppression of GABAA-receptor-mediated IPSPs after epileptiform burst discharges in CA1 pyramidal cells. J Neurophysiol 79: 659-669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
