Memory CD8 T-cell differentiation during viral infection
- PMID: 15140950
- PMCID: PMC415833
- DOI: 10.1128/JVI.78.11.5535-5545.2004
Memory CD8 T-cell differentiation during viral infection
Figures
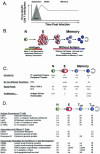


References
-
- Aandahl, E. M., J. K. Sandberg, K. P. Beckerman, K. Tasken, W. J. Moretto, and D. F. Nixon. 2003. CD7 is a differentiation marker that identifies multiple CD8 T cell effector subsets. J. Immunol. 170:2349-2355. - PubMed
-
- Abendroth, A., and A. Arvin. 1999. Varicella-zoster virus immune evasion. Immunol. Rev. 168:143-156. - PubMed
-
- Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. - PubMed
-
- Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

