Context-dependent effects of L domains and ubiquitination on viral budding
- PMID: 15140952
- PMCID: PMC415830
- DOI: 10.1128/JVI.78.11.5554-5563.2004
Context-dependent effects of L domains and ubiquitination on viral budding
Abstract
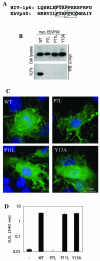
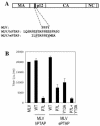
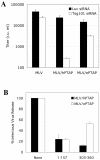
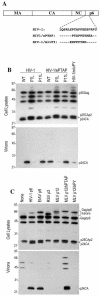
Many enveloped viruses encode late assembly domains, or L domains, that facilitate virion egress. PTAP-type L domains act by recruiting the ESCRT-I (endosomal sorting complex required for transport I) component Tsg101, and YPXL/LXXLF-type L domains recruit AIP-1/ALIX, both of which are class E vacuolar protein sorting (VPS) factors, normally required for the generation of vesicles within endosomes. The binding cofactors for PPXY-type L domains have not been unambiguously resolved but may include Nedd4-like ubiquitin ligases. Largely because they act as autonomous binding sites for host factors, L domains are generally transferable and active in a context-independent manner. Ebola virus matrix protein (EbVP40) contains two overlapping L-domain motifs within the sequence ILPTAPPEYMEA. Here, we show that both motifs are required for efficient EbVP40 budding. However, upon transplantation into two different retroviral contexts, the relative contributions of the PTAP and PPEY motifs differ markedly. In a murine leukemia virus carrying the EbVP40 sequence, both motifs contributed to overall L domain activity, and budding proceeded in a partly Tsg101-independent manner. Conversely, when transplanted into the context of human immunodeficiency virus type 1 (HIV-1), EbVP40 L-domain activity was entirely due to a PTAP-Tsg101 interaction. In fact, a number of PPXY-type L domains were inactive in the context of HIV-1. Surprisingly, PTAP and YPXL-type L domains that simulated HIV-1 budding reduced the amount of ubiquitin conjugated to Gag, while inactive PPXY-type L domains increased Gag ubiquitination. These observations suggest that active L domains recruit deubiquitinating enzymes as a consequence of class E VPS factor recruitment. Moreover, context-dependent L-domain function may reflect distinct requirements for host functions during the morphogenesis of different viral particles or the underlying presence of additional, as yet undiscovered L domains.
Figures
Similar articles
-
HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress.Nat Med. 2001 Dec;7(12):1313-9. doi: 10.1038/nm1201-1313. Nat Med. 2001. PMID: 11726971
-
Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4.J Virol. 2003 Feb;77(3):1812-9. doi: 10.1128/jvi.77.3.1812-1819.2003. J Virol. 2003. PMID: 12525615 Free PMC article.
-
ALIX Rescues Budding of a Double PTAP/PPEY L-Domain Deletion Mutant of Ebola VP40: A Role for ALIX in Ebola Virus Egress.J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S138-45. doi: 10.1093/infdis/jiu838. Epub 2015 Mar 18. J Infect Dis. 2015. PMID: 25786915 Free PMC article.
-
[Envelope virus assembly and budding].Uirusu. 2010 Jun;60(1):105-13. doi: 10.2222/jsv.60.105. Uirusu. 2010. PMID: 20848870 Review. Japanese.
-
Retrovirus budding.Virus Res. 2004 Dec;106(2):87-102. doi: 10.1016/j.virusres.2004.08.007. Virus Res. 2004. PMID: 15567490 Review.
Cited by
-
Compound FC-10696 Inhibits Egress of Marburg Virus.Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0008621. doi: 10.1128/AAC.00086-21. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33846137 Free PMC article.
-
Ubiquitin-dependent virus particle budding without viral protein ubiquitination.Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):20031-6. doi: 10.1073/pnas.0708002104. Epub 2007 Dec 3. Proc Natl Acad Sci U S A. 2007. PMID: 18056634 Free PMC article.
-
Regulation of Tsg101 expression by the steadiness box: a role of Tsg101-associated ligase.Mol Biol Cell. 2008 Feb;19(2):754-63. doi: 10.1091/mbc.e07-09-0957. Epub 2007 Dec 12. Mol Biol Cell. 2008. PMID: 18077552 Free PMC article.
-
Viruses go modular.J Biol Chem. 2020 Apr 3;295(14):4604-4616. doi: 10.1074/jbc.REV119.012414. Epub 2020 Feb 28. J Biol Chem. 2020. PMID: 32111739 Free PMC article. Review.
-
The nucleocapsid region of human immunodeficiency virus type 1 Gag assists in the coordination of assembly and Gag processing: role for RNA-Gag binding in the early stages of assembly.J Virol. 2009 Aug;83(15):7718-27. doi: 10.1128/JVI.00099-09. Epub 2009 May 20. J Virol. 2009. PMID: 19457986 Free PMC article.
References
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. - PubMed
-
- Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland, and S. D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:283-289. - PubMed
-
- Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

