Parvovirus infection of cells by using variants of the feline transferrin receptor altering clathrin-mediated endocytosis, membrane domain localization, and capsid-binding domains
- PMID: 15140957
- PMCID: PMC415789
- DOI: 10.1128/JVI.78.11.5601-5611.2004
Parvovirus infection of cells by using variants of the feline transferrin receptor altering clathrin-mediated endocytosis, membrane domain localization, and capsid-binding domains
Abstract
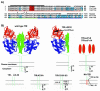
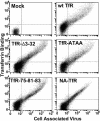
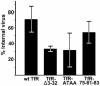
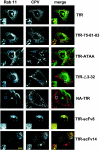
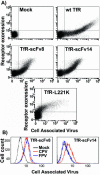
The feline and canine transferrin receptors (TfRs) bind canine parvovirus to host cells and mediate rapid capsid uptake and infection. The TfR and its ligand transferrin have well-described pathways of endocytosis and recycling. Here we tested several receptor-dependent steps in infection for their role in virus infection of cells. Deletions of cytoplasmic sequences or mutations of the Tyr-Thr-Arg-Phe internalization motif reduced the rate of receptor uptake from the cell surface, while polar residues introduced into the transmembrane sequence resulted in increased degradation of transferrin. However, the mutant receptors still mediated efficient virus infection. In contrast, replacing the cytoplasmic and transmembrane sequences of the feline TfR with those of the influenza virus neuraminidase (NA) resulted in a receptor that bound and endocytosed the capsid but did not mediate viral infection. This chimeric receptor became localized to detergent-insoluble membrane domains. To test the effect of structural virus receptor interaction on infection, two chimeric receptors were prepared which contained antibody-variable domains that bound the capsid in place of the TfR ectodomain. These chimeric receptors bound CPV capsids and mediated uptake but did not result in cell infection. Adding soluble feline TfR ectodomain to the virus during that uptake did not allow infection.
Figures
Similar articles
-
Purified feline and canine transferrin receptors reveal complex interactions with the capsids of canine and feline parvoviruses that correspond to their host ranges.J Virol. 2006 Sep;80(17):8482-92. doi: 10.1128/JVI.00683-06. J Virol. 2006. PMID: 16912298 Free PMC article.
-
Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses.J Virol. 2003 Aug;77(16):8915-23. doi: 10.1128/jvi.77.16.8915-8923.2003. J Virol. 2003. PMID: 12885908 Free PMC article.
-
Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection.J Virol. 2010 May;84(10):4969-78. doi: 10.1128/JVI.02623-09. Epub 2010 Mar 3. J Virol. 2010. PMID: 20200243 Free PMC article.
-
[Evolution and host variation of the canine parvovirus: molecular basis for the development of a new virus].Berl Munch Tierarztl Wochenschr. 2004 Mar-Apr;117(3-4):130-5. Berl Munch Tierarztl Wochenschr. 2004. PMID: 15046459 Review. German.
-
Structures and functions of parvovirus capsids and the process of cell infection.Curr Top Microbiol Immunol. 2010;343:149-76. doi: 10.1007/82_2010_33. Curr Top Microbiol Immunol. 2010. PMID: 20397069 Review.
Cited by
-
Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking.J Virol. 2009 Oct;83(20):10504-14. doi: 10.1128/JVI.00295-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656887 Free PMC article.
-
Soluble form of canine transferrin receptor inhibits canine parvovirus infection in vitro and in vivo.Biomed Res Int. 2013;2013:172479. doi: 10.1155/2013/172479. Epub 2013 Sep 8. Biomed Res Int. 2013. PMID: 24089666 Free PMC article.
-
Evaluation of two internalizing carcinoembryonic antigen reporter genes for molecular imaging.Mol Imaging Biol. 2011 Jun;13(3):526-535. doi: 10.1007/s11307-010-0375-0. Mol Imaging Biol. 2011. PMID: 20628903 Free PMC article.
-
Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus.J Virol. 2010 Aug;84(15):7782-92. doi: 10.1128/JVI.00479-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484503 Free PMC article.
-
Detecting small changes and additional peptides in the canine parvovirus capsid structure.J Virol. 2008 Nov;82(21):10397-407. doi: 10.1128/JVI.00972-08. Epub 2008 Aug 13. J Virol. 2008. PMID: 18701590 Free PMC article.
References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Barbis, D. P., S.-F. Chang, and C. R. Parrish. 1992. Mutations adjacent to the dimple of canine parvovirus capsid structure affect sialic acid binding. Virology 191:301-308. - PubMed
-
- Bartlett, J. S., J. Kleinschmidt, R. C. Boucher, and R. J. Samulski. 1999. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′gamma)2 antibody. Nat. Biotechnol. 17:181-186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

