Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase
- PMID: 15140959
- PMCID: PMC415832
- DOI: 10.1128/JVI.78.11.5619-5632.2004
Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase
Abstract
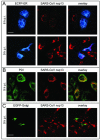
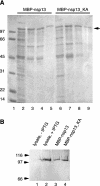
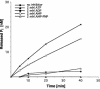
Severe acute respiratory syndrome coronavirus (SARS-CoV), a newly identified group 2 coronavirus, is the causative agent of severe acute respiratory syndrome, a life-threatening form of pneumonia in humans. Coronavirus replication and transcription are highly specialized processes of cytoplasmic RNA synthesis that localize to virus-induced membrane structures and were recently proposed to involve a complex enzymatic machinery that, besides RNA-dependent RNA polymerase, helicase, and protease activities, also involves a series of RNA-processing enzymes that are not found in most other RNA virus families. Here, we characterized the enzymatic activities of a recombinant form of the SARS-CoV helicase (nonstructural protein [nsp] 13), a superfamily 1 helicase with an N-terminal zinc-binding domain. We report that nsp13 has both RNA and DNA duplex-unwinding activities. SARS-CoV nsp13 unwinds its substrates in a 5'-to-3' direction and features a remarkable processivity, allowing efficient strand separation of extended regions of double-stranded RNA and DNA. Characterization of the nsp13-associated (deoxy)nucleoside triphosphatase ([dNTPase) activities revealed that all natural nucleotides and deoxynucleotides are substrates of nsp13, with ATP, dATP, and GTP being hydrolyzed slightly more efficiently than other nucleotides. Furthermore, we established an RNA 5'-triphosphatase activity for the SARS-CoV nsp13 helicase which may be involved in the formation of the 5' cap structure of viral RNAs. The data suggest that the (d)NTPase and RNA 5'-triphosphatase activities of nsp13 have a common active site. Finally, we established that, in SARS-CoV-infected Vero E6 cells, nsp13 localizes to membranes that appear to be derived from the endoplasmic reticulum and are the likely site of SARS-CoV RNA synthesis.
Figures





References
-
- Anand, K., J. Ziebuhr, P. Wadhwani, J. R. Mesters, and R. Hilgenfeld. 2003. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300:1763-1767. - PubMed
-
- Bird, L. E., H. S. Subramanya, and D. B. Wigley. 1998. Helicases: a unifying structural theme? Curr. Opin. Struct. Biol. 8:14-18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

