Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively
- PMID: 15140960
- PMCID: PMC415790
- DOI: 10.1128/JVI.78.11.5633-5641.2004
Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively
Abstract
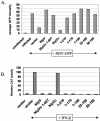
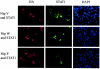
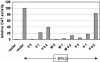
In previous reports it was demonstrated that the Nipah virus V and W proteins have interferon (IFN) antagonist activity due to their ability to block signaling from the IFN-alpha/beta receptor (J. J. Rodriguez, J. P. Parisien, and C. M. Horvath, J. Virol. 76:11476-11483, 2002; M. S. Park et al., J. Virol. 77:1501-1511, 2003). The V, W, and P proteins are all encoded by the same viral gene and share an identical 407-amino-acid N-terminal region but have distinct C-terminal sequences. We now show that the P protein also has anti-IFN function, confirming that the common N-terminal domain is responsible for the antagonist activity. Truncation of this N-terminal domain revealed that amino acids 50 to 150 retain the ability to block IFN and to bind STAT1, a key component of the IFN signaling pathway. Subcellular localization studies demonstrate that the V and P proteins are predominantly cytoplasmic whereas the W protein is localized to the nucleus. In all cases, STAT1 colocalizes with the corresponding Nipah virus protein. These interactions are sufficient to inhibit STAT1 activation, as demonstrated by the lack of STAT1 phosphorylation on tyrosine 701 in IFN-stimulated cells expressing P, V, or W. Therefore, despite their common STAT1-binding domain, the Nipah virus V and P proteins act by retaining STAT1 in the cytoplasm while the W protein sequesters STAT1 in the nucleus, creating both a cytoplasmic and a nuclear block for STAT1. We also show that the IFN antagonist activity of the P protein is not as strong as that of V or W, perhaps explaining why Nipah virus has evolved to express these two edited products.
Figures






Similar articles
-
The nucleocytoplasmic rabies virus P protein counteracts interferon signaling by inhibiting both nuclear accumulation and DNA binding of STAT1.J Virol. 2007 Apr;81(8):4255-63. doi: 10.1128/JVI.01930-06. Epub 2007 Feb 7. J Virol. 2007. PMID: 17287281 Free PMC article.
-
Interactions of the Nipah Virus P, V, and W Proteins across the STAT Family of Transcription Factors.mSphere. 2020 Dec 16;5(6):e00449-20. doi: 10.1128/mSphere.00449-20. mSphere. 2020. PMID: 33328346 Free PMC article.
-
Dephosphorylation failure of tyrosine-phosphorylated STAT1 in IFN-stimulated Sendai virus C protein-expressing cells.Virology. 2002 Feb 15;293(2):205-9. doi: 10.1006/viro.2001.1250. Virology. 2002. PMID: 11886239
-
The ins and outs of STAT1 nuclear transport.Sci STKE. 2003 Aug 12;2003(195):RE13. doi: 10.1126/stke.2003.195.re13. Sci STKE. 2003. PMID: 12915721 Review.
-
Host evasion by emerging paramyxoviruses: Hendra virus and Nipah virus v proteins inhibit interferon signaling.Viral Immunol. 2004;17(2):210-9. doi: 10.1089/0882824041310568. Viral Immunol. 2004. PMID: 15279700 Review.
Cited by
-
Nipah virus matrix protein: expert hacker of cellular machines.FEBS Lett. 2016 Aug;590(15):2494-511. doi: 10.1002/1873-3468.12272. Epub 2016 Jul 12. FEBS Lett. 2016. PMID: 27350027 Free PMC article. Review.
-
Nipah Virus C and W Proteins Contribute to Respiratory Disease in Ferrets.J Virol. 2016 Jun 24;90(14):6326-6343. doi: 10.1128/JVI.00215-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147733 Free PMC article.
-
Molecular Mechanisms of Innate Immune Inhibition by Non-Segmented Negative-Sense RNA Viruses.J Mol Biol. 2016 Aug 28;428(17):3467-82. doi: 10.1016/j.jmb.2016.07.017. Epub 2016 Jul 31. J Mol Biol. 2016. PMID: 27487481 Free PMC article. Review.
-
Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling.Cell Host Microbe. 2016 Jun 8;19(6):882-90. doi: 10.1016/j.chom.2016.05.009. Epub 2016 May 19. Cell Host Microbe. 2016. PMID: 27212660 Free PMC article.
-
Two domains of the V protein of virulent canine distemper virus selectively inhibit STAT1 and STAT2 nuclear import.J Virol. 2010 Jul;84(13):6328-43. doi: 10.1128/JVI.01878-09. Epub 2010 Apr 28. J Virol. 2010. PMID: 20427537 Free PMC article.
References
-
- Basler, C. F., A. H. Reid, J. K. Dybing, T. A. Janczewski, T. G. Fanning, H. Zheng, M. Salvatore, M. L. Perdue, D. E. Swayne, A. Garcia-Sastre, P. Palese, and J. K. Taubenberger. 2001. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc. Natl. Acad. Sci. USA 98:2746-2751. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

