Murine coronavirus replication induces cell cycle arrest in G0/G1 phase
- PMID: 15140963
- PMCID: PMC415820
- DOI: 10.1128/JVI.78.11.5658-5669.2004
Murine coronavirus replication induces cell cycle arrest in G0/G1 phase
Abstract
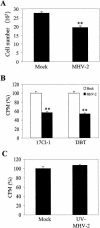
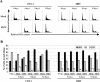
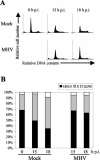
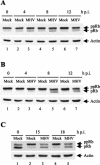
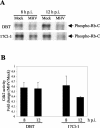
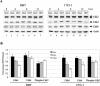
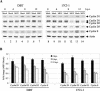
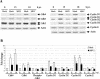
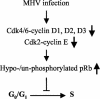
Mouse hepatitis virus (MHV) replication in actively growing DBT and 17Cl-1 cells resulted in the inhibition of host cellular DNA synthesis and the accumulation of infected cells in the G(0)/G(1) phase of the cell cycle. UV-irradiated MHV failed to inhibit host cellular DNA synthesis. MHV infection in quiescent 17Cl-1 cells that had been synchronized in the G(0) phase by serum deprivation prevented infected cells from entering the S phase after serum stimulation. MHV replication inhibited hyperphosphorylation of the retinoblastoma protein (pRb), the event that is necessary for cell cycle progression through late G(1) and into the S phase. While the amounts of the cellular cyclin-dependent kinase (Cdk) inhibitors p21(Cip1), p27(Kip1), and p16(INK4a) did not change in infected cells, MHV infection in asynchronous cultures induced a clear reduction in the amounts of Cdk4 and G(1) cyclins (cyclins D1, D2, D3, and E) in both DBT and 17Cl-1 cells and a reduction in Cdk6 levels in 17Cl-1 cells. Infection also resulted in a decrease in Cdk2 activity in both cell lines. MHV infection in quiescent 17Cl-1 cells prevented normal increases in Cdk4, Cdk6, cyclin D1, and cyclin D3 levels after serum stimulation. The amounts of cyclin D2 and cyclin E were not increased significantly after serum stimulation in mock-infected cells, whereas they were decreased in MHV-infected cells, suggesting the possibility that MHV infection may induce cyclin D2 and cyclin E degradation. Our data suggested that a reduction in the amounts of G(1) cyclin-Cdk complexes in MHV-infected cells led to a reduction in Cdk activities and insufficient hyperphosphorylation of pRb, resulting in inhibition of the cell cycle in the G(0)/G(1) phase.
Figures

References
-
- Bonneau, A. M., and N. Sonenberg. 1987. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J. Biol. Chem. 262:11134-11139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

