IGFBP-3, a marker of cellular senescence, is overexpressed in human papillomavirus-immortalized cervical cells and enhances IGF-1-induced mitogenesis
- PMID: 15140969
- PMCID: PMC415828
- DOI: 10.1128/JVI.78.11.5720-5727.2004
IGFBP-3, a marker of cellular senescence, is overexpressed in human papillomavirus-immortalized cervical cells and enhances IGF-1-induced mitogenesis
Abstract
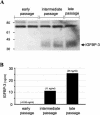
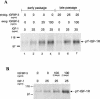
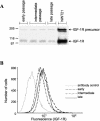
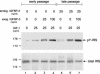
Human ectocervical cells, following retroviral transduction with the human papillomavirus type 16 E6/E7 oncogenes, are altered in their array of transcribed cellular genes, including increased mRNA for the insulin-like growth factor binding protein 3 (IGFBP-3). IGFBP-3 expression is associated with cellular senescence, and its addition to many cell types inhibits growth or induces apoptosis. By immunoblotting and enzyme-linked immunosorbent assay methods, we demonstrate that late-passage, immortalized E6/E7-transduced cells secrete high levels of IGFBP-3 (25 ng/ml), which represent a 500-fold increase compared to levels in early-passage, nonimmortalized transduced cells (<0.05 ng/ml). Concomitantly, these late-passage cervical cells exhibit an increase in sensitivity to IGF-1, including enhanced phosphorylation of the IGF receptor (IGF-R) and insulin receptor substrate as well as increased DNA synthesis (5-fold) and cell proliferation (3.7-fold). However, there was no change in the level of IGF-R in these cells (surface or total), and the cells did not synthesize IGF-1, indicating that these arms of the IGF pathway were independently regulated and not responsible for the augmented signaling. Consistent with a causal relationship between IGFBP-3 expression and enhanced IGF-1 responses, we found that early-passage cells could be converted to the late-passage, IGF-1-responsive phenotype by preincubation with IGFBP-3. Thus, in contrast to findings with some cell types, IGFBP-3 expression in cervical cells is associated with augmented IGF-1 signaling and cell proliferation and correlates with the timing of cellular immortalization.
Figures
References
-
- Andreatta-Van Leyen, S., J. R. Hembree, and R. L. Eckert. 1994. Regulation of insulin-like growth factor 1 binding protein 3 levels by epidermal growth factor and retinoic acid in cervical epithelial cells. J. Cell. Physiol. 160:265-274. - PubMed
-
- Baege, A. C., A. Berger, R. Schlegel, T. Veldman, and R. Schlegel. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251-1257. - PMC - PubMed
-
- Barreca, A., M. De Luca, P. Del Monte, S. Bondanza, G. Damonte, G. Cariola, E. Di Marco, G. Giordano, R. Cancedda, and F. Minuto. 1992. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J. Cell. Physiol. 151:262-268. - PubMed
-
- Baserga, R. 1995. The insulin-like growth factor 1 receptor: a key to tumor growth? Cancer Res. 55:249-252. - PubMed
-
- Baserga, R., A. Hongo, M. Rubin, M. Prisco, and B. Valentinis. 1997. The IGF-1 receptor in cell growth, transformation and apoptosis. Biochem. Biophys. Acta 1332:105-126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

