Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity
- PMID: 15140972
- PMCID: PMC415815
- DOI: 10.1128/JVI.78.11.5745-5755.2004
Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity
Abstract
The human immunodeficiency virus (HIV) protein Nef has been shown to increase the infectivity of HIV at an early point during infection. Since Nef is known to interact with proteins involved in actin cytoskeleton rearrangements, we tested the possibility that Nef may enhance HIV infectivity via a mechanism that involves the actin cytoskeleton. We find that disruption of the actin cytoskeleton complements the Nef infectivity defect. The ability of disruption of the actin cytoskeleton to complement the Nef defect was specific to envelopes that fuse at the cell surface, including a variety of HIV envelopes and the murine leukemia virus amphotropic envelope. In contrast, the infectivity of HIV virions pseudotyped to enter cells via endocytosis, which is known to complement the HIV Nef infectivity defect and can naturally penetrate the cortical actin barrier, was not altered by actin cytoskeleton disruption. The results presented here suggest that Nef functions to allow the HIV genome to penetrate the cortical actin network, a known barrier for intracellular parasitic organisms.
Figures

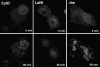
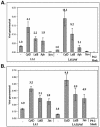
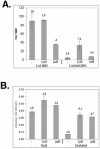




References
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Anderson, J. L., and T. J. Hope. 2003. Recent insights into HIV accessory proteins. Curr. Infect. Dis. Rep. 5:439-450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

