The dominant-negative herpes simplex virus type 1 (HSV-1) recombinant CJ83193 can serve as an effective vaccine against wild-type HSV-1 infection in mice
- PMID: 15140973
- PMCID: PMC415800
- DOI: 10.1128/JVI.78.11.5756-5765.2004
The dominant-negative herpes simplex virus type 1 (HSV-1) recombinant CJ83193 can serve as an effective vaccine against wild-type HSV-1 infection in mice
Abstract
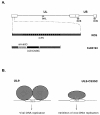
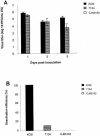
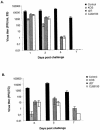
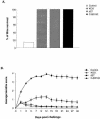
By selectively regulating the expression of the trans-dominant-negative mutant polypeptide UL9-C535C, of herpes simplex virus type 1 (HSV-1) origin binding protein UL9 with the tetracycline repressor (tetR)-mediated gene switch, we recently generated a novel replication-defective and anti-HSV-specific HSV-1 recombinant, CJ83193. The UL9-C535C peptides expressed by CJ83193 can function as a potent intracellular therapy against its own replication, as well as the replication of wild-type HSV-1 and HSV-2 in coinfected cells. In this report, we demonstrate that CJ83193 cannot initiate acute productive infection in corneas of infected mice nor can it reactivate from trigeminal ganglia of mice latently infected by CJ83193 in a mouse ocular model. Given that CJ83193 is capable of expressing the viral alpha, beta, and gamma1 genes but little or no gamma2 genes, we tested the vaccine potential of CJ83193 against HSV-1 infection in a mouse ocular model. Our studies showed that immunization with CJ83193 significantly reduced the yields of challenge HSV in the eyes and trigeminal ganglia on days 3, 5, and 7 postchallenge. Like in mice immunized with the wild-type HSV-1 strain KOS, immunization of mice with CJ83193 prevents the development of keratitis and encephalitis induced by corneal challenge with wild-type HSV-1 strain mP. Delayed-type hypersensitivity (DTH) assays demonstrate that CJ83193 can elicit durable cell-mediated immunity at the same level as that of wild-type HSV-1 and is more effective than that induced by d27, an HSV-1 ICP27 deletion mutant. Moreover, mice immunized with CJ83193 developed strong, durable HSV-1-neutralizing antibodies at levels at least twofold higher than those induced by d27. The results presented in this report have shed new light on the development of effective HSV viral vaccines that encode a unique safety mechanism capable of inhibiting the mutant's own replication and that of wild-type virus.
Figures


Similar articles
-
Inhibition of herpes simplex virus type 2 (HSV-2) viral replication by the dominant negative mutant polypeptide of HSV-1 origin binding protein.Antiviral Res. 2002 Feb;53(2):127-33. doi: 10.1016/s0166-3542(01)00207-8. Antiviral Res. 2002. PMID: 11750938
-
A novel anti-herpes simplex virus type 1-specific herpes simplex virus type 1 recombinant.Hum Gene Ther. 1999 Jul 20;10(11):1811-8. doi: 10.1089/10430349950017491. Hum Gene Ther. 1999. PMID: 10446921
-
High-level expression of glycoprotein D by a dominant-negative HSV-1 virus augments its efficacy as a vaccine against HSV-1 infection.J Invest Dermatol. 2009 May;129(5):1174-84. doi: 10.1038/jid.2008.349. Epub 2008 Nov 13. J Invest Dermatol. 2009. PMID: 19005489 Free PMC article.
-
Recent advances in vaccine development for herpes simplex virus types I and II.Hum Vaccin Immunother. 2013 Apr;9(4):729-35. doi: 10.4161/hv.23289. Epub 2013 Feb 26. Hum Vaccin Immunother. 2013. PMID: 23442925 Free PMC article. Review.
-
Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back.Front Immunol. 2022 Apr 25;13:849515. doi: 10.3389/fimmu.2022.849515. eCollection 2022. Front Immunol. 2022. PMID: 35547736 Free PMC article. Review.
Cited by
-
Development of a regulatable oncolytic herpes simplex virus type 1 recombinant virus for tumor therapy.J Virol. 2010 Aug;84(16):8163-71. doi: 10.1128/JVI.00059-10. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519407 Free PMC article.
-
Immunization with a dominant-negative recombinant Herpes Simplex Virus (HSV) type 1 protects against HSV-2 genital disease in guinea pigs.BMC Microbiol. 2010 Jun 3;10:163. doi: 10.1186/1471-2180-10-163. BMC Microbiol. 2010. PMID: 20525279 Free PMC article.
-
Attenuated phenotypes and analysis of a herpes simplex virus 1 strain with partial deletion of the UL7, UL41 and LAT genes.Virol Sin. 2017 Oct;32(5):404-414. doi: 10.1007/s12250-017-3947-1. Epub 2017 Sep 29. Virol Sin. 2017. PMID: 28971351 Free PMC article.
-
[Vaccines against herpex simplex virus infections. What's new].Hautarzt. 2007 May;58(5):465-6. doi: 10.1007/s00105-007-1345-6. Hautarzt. 2007. PMID: 17450340 German. No abstract available.
-
Single dose of glycoprotein K (gK)-deleted HSV-1 live-attenuated virus protects mice against lethal vaginal challenge with HSV-1 and HSV-2 and induces lasting T cell memory immune responses.Virol J. 2013 Oct 28;10:317. doi: 10.1186/1743-422X-10-317. Virol J. 2013. PMID: 24165088 Free PMC article.
References
-
- Bourne, N., G. N. Milligan, M. R. Schleiss, D. I. Bernstein, and L. R. Stanberry. 1996. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine 14:1230-1234. - PubMed
-
- Boursnell, M. E., C. Entwisle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, R. Jennings, D. Ni Challanain, I. Sobek, S. C. Inglis, and C. S. McLean. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. - PMC - PubMed
-
- Brehm, M. A., R. H. Bonneau, D. M. Knipe, and S. S. Tevethia. 1997. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J. Virol. 71:3534-3544. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

