Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site
- PMID: 15140989
- PMCID: PMC415842
- DOI: 10.1128/JVI.78.11.5913-5922.2004
Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site
Abstract
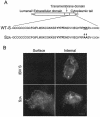
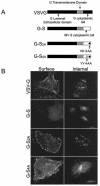
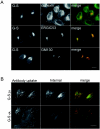
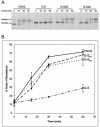
Coronavirus budding at the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) requires accumulation of the viral envelope proteins at this point in the secretory pathway. Here we demonstrate that the spike (S) protein from the group 3 coronavirus infectious bronchitis virus (IBV) contains a canonical dilysine endoplasmic reticulum retrieval signal (-KKXX-COOH) in its cytoplasmic tail. This signal can retain a chimeric reporter protein in the ERGIC and when mutated allows transport of the full-length S protein as well as the chimera to the plasma membrane. Interestingly, the IBV S protein also contains a tyrosine-based endocytosis signal in its cytoplasmic tail, suggesting that any S protein that escapes the ERGIC will be rapidly endocytosed when it reaches the plasma membrane. We also identified a novel dibasic motif (-KXHXX-COOH) in the cytoplasmic tails of S proteins from group 1 coronaviruses and from the newly identified coronavirus implicated in severe acute respiratory syndrome. This dibasic motif also retained a reporter protein in the ERGIC, similar to the dilysine motif in IBV S. The cytoplasmic tails of S proteins from group 2 coronaviruses lack an intracellular localization signal. The inherent differences in S-protein trafficking could point to interesting variations in pathogenesis of coronaviruses, since increased levels of surface S protein could promote syncytium formation and direct cell-to-cell spread of the infection.
Figures
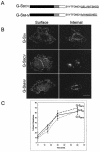
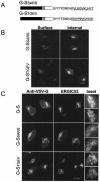
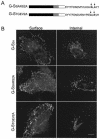
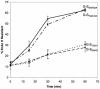
Similar articles
-
The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein.J Virol. 2007 Mar;81(5):2418-28. doi: 10.1128/JVI.02146-06. Epub 2006 Dec 13. J Virol. 2007. PMID: 17166901 Free PMC article.
-
The cytoplasmic tail of IBV spike mediates intracellular retention via interaction with COPI-coated vesicles in retrograde trafficking.J Virol. 2025 Feb 25;99(2):e0216424. doi: 10.1128/jvi.02164-24. Epub 2025 Jan 22. J Virol. 2025. PMID: 39840971 Free PMC article.
-
A single tyrosine in the severe acute respiratory syndrome coronavirus membrane protein cytoplasmic tail is important for efficient interaction with spike protein.J Virol. 2010 Feb;84(4):1891-901. doi: 10.1128/JVI.02458-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007283 Free PMC article.
-
Incorporation of spike and membrane glycoproteins into coronavirus virions.Viruses. 2015 Apr 3;7(4):1700-25. doi: 10.3390/v7041700. Viruses. 2015. PMID: 25855243 Free PMC article. Review.
-
The avian coronavirus spike protein.Virus Res. 2014 Dec 19;194:37-48. doi: 10.1016/j.virusres.2014.10.009. Epub 2014 Oct 17. Virus Res. 2014. PMID: 25451062 Free PMC article. Review.
Cited by
-
Analysis of glycosylation and disulfide bonding of wild-type SARS-CoV-2 spike glycoprotein.bioRxiv [Preprint]. 2021 Apr 1:2021.04.01.438120. doi: 10.1101/2021.04.01.438120. bioRxiv. 2021. Update in: J Virol. 2022 Feb 9;96(3):e0162621. doi: 10.1128/JVI.01626-21. PMID: 33821278 Free PMC article. Updated. Preprint.
-
Transcriptional regulation of RNA3 of infectious bronchitis virus.Adv Exp Med Biol. 2006;581:109-12. doi: 10.1007/978-0-387-33012-9_19. Adv Exp Med Biol. 2006. PMID: 17037515 Free PMC article. No abstract available.
-
The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein.J Virol. 2007 Mar;81(5):2418-28. doi: 10.1128/JVI.02146-06. Epub 2006 Dec 13. J Virol. 2007. PMID: 17166901 Free PMC article.
-
A method for the generation of pseudovirus particles bearing SARS coronavirus spike protein in high yields.Cell Struct Funct. 2022 Jun 25;47(1):43-53. doi: 10.1247/csf.21047. Epub 2022 Apr 28. Cell Struct Funct. 2022. PMID: 35491102 Free PMC article.
-
Cytoplasmic Tail Truncation Stabilizes S1-S2 Association and Enhances S Protein Incorporation into SARS-CoV-2 Pseudovirions.J Virol. 2023 Mar 30;97(3):e0165022. doi: 10.1128/jvi.01650-22. Epub 2023 Feb 15. J Virol. 2023. PMID: 36790205 Free PMC article.
References
-
- Andersson, H., F. Kappeler, and H. P. Hauri. 1999. Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem. 274:15080-15084. - PubMed
-
- Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

