Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV
- PMID: 15141000
- PMCID: PMC415805
- DOI: 10.1128/JVI.78.11.6024-6032.2004
Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV
Abstract
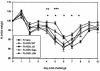
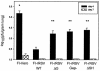
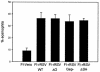
Following respiratory syncytial virus (RSV) challenge, mice immunized with RSV G or with formalin-inactivated RSV (FI-RSV) exhibit severe disease associated with type 2 cytokine production and pulmonary eosinophilia. This has led to the proposal that the presence of RSV G is the factor in FI-RSV that induces disease-enhancing T-cell responses. Therefore, we evaluated the role of RSV G and its immunodominant region in the induction of aberrant immune responses during FI-RSV immunization. BALB/c mice were immunized with FI preparations of wild-type (wt) RSV or recombinant RSV (rRSV) containing deletions of (i) the entire G gene, (ii) the region of the G gene encoding amino acids 187 to 197 of the immunodominant region, or (iii) the entire SH gene. After challenge, illness, RSV titers, cytokine levels, and pulmonary eosinophilia were measured. Peak RSV titers postchallenge were significantly greater in mice immunized with FI preparations of the deletion viruses than in those immunized with FI-rRSV wt, suggesting that the absence of G or SH in FI-RSV reduced its protective efficacy. Deletion of G or its epitope did not reduce illness, cytokine production, or eosinophilia relative to that in mice immunized with FI-rRSV wt. While cytokine levels and eosinophilia were similar, illness was reduced in mice immunized with SH-deleted FI-RSV. These data suggest that G-specific immune responses may be important for vaccine-induced protection and are not solely the basis for FI-RSV vaccine-enhanced illness. These data suggest that the method of RSV antigen delivery, rather than the protein composition, influences the phenotype of the induced immune responses and that RSV G should not necessarily be excluded from potential vaccine strategies.
Figures

References
-
- Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473-2480. - PubMed
-
- Bukreyev, A., S. S. Whitehead, B. R. Murphy, and P. L. Collins. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J. Virol. 71:8973-8982. - PMC - PubMed
-
- Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 92:11563-11567. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

