Catalysis-based enantioselective total synthesis of the macrocyclic spermidine alkaloid isooncinotine
- PMID: 15141085
- PMCID: PMC514416
- DOI: 10.1073/pnas.0401322101
Catalysis-based enantioselective total synthesis of the macrocyclic spermidine alkaloid isooncinotine
Abstract
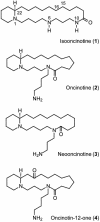
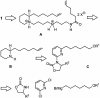
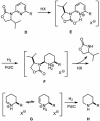
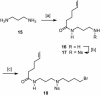
A concise and efficient total synthesis of the spermidine alkaloid (-)-isooncinotine (1) incorporating a 22-membered lactam ring is outlined. The approach is largely catalysis-based, involving a selective iron-catalyzed alkyl-aryl cross-coupling reaction of a difunctionalized pyridine substrate, a heterogeneous asymmetric hydrogenation step to set the chiral center of the target, and a highly integrated ring-closing metathesis/hydrogenation sequence to forge the saturated macrocyclic edifice in a single operation.
Figures

References
-
- Morris, D. R. & Marton, L. J. (1981) Polyamines in Biology and Medicine (Dekker, New York).
-
- Guggisberg, A. & Hesse, M. (1998) Alkaloids 50, 219-256.
-
- Karigiannis, G. & Papaioannou, D. (2000) Eur. J. Org. Chem., 1841-1863.
-
- Frydman, B., Bhattacharya, S., Sarkar, A., Drandarov, K., Chesnov, S., Guggisberg, A., Popaj, K., Yurdakul, A., Hesse, M., Basu, H. S., et al. (2004) J. Med. Chem. 47, 1051-1059. - PubMed
-
- Badawi, M. M., Guggisberg, A., van den Broek, P., Hesse, M. & Schmid, H. (1968) Helv. Chim. Acta 51, 1813-1817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

