Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets
- PMID: 15141093
- PMCID: PMC419570
- DOI: 10.1073/pnas.0305683101
Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets
Abstract
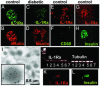
High concentrations of glucose induce beta cell production of IL-1beta, leading to impaired beta cell function and apoptosis in human pancreatic islets. IL-1 receptor antagonist (IL-1Ra) is a naturally occurring antagonist of IL-1beta and protects cultured human islets from glucotoxicity. Therefore, the balance of IL-1beta and IL-1Ra may play a crucial role in the pathogenesis of diabetes. In the present study, we observed expression of IL-1Ra in human pancreatic beta cells of nondiabetic individuals, which was decreased in tissue sections of type 2 diabetic patients. In vitro, chronic exposure of human islets to leptin, a hormone secreted by adipocytes, decreased beta cell production of IL-1Ra and induced IL-1beta release from the islet preparation, leading to impaired beta cell function, caspase-3 activation, and apoptosis. Exogenous addition of IL-1Ra protected cultured human islets from the deleterious effects of leptin. Antagonizing IL-1Ra by introduction of small interfering RNA to IL-1Ra into human islets led to caspase-3 activation, DNA fragmentation, and impaired beta cell function. Moreover, siIL-1Ra enhanced glucose-induced beta cell apoptosis. These findings demonstrate expression of IL-1Ra in the human beta cell, providing localized protection against leptin- and glucose-induced islet IL-1beta.
Figures

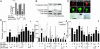
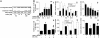
References
-
- Bonner-Weir, S. (2000) J. Mol. Endocrinol. 24, 297–302. - PubMed
-
- Butler, A. E., Janson, J., Bonner-Weir, S., Ritzel, R., Rizza, R. A. & Butler, P. C. (2003) Diabetes 52, 102–110. - PubMed
-
- Cerasi, E. (1995) Diabetologia 38, 992–997. - PubMed
-
- Donath, M. Y., Gross, D. J., Cerasi, E. & Kaiser, N. (1999) Diabetes 48, 738–744. - PubMed
-
- Leroith, D. (2002) Am. J. Med. 113 Suppl 6a, 3s–11s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

