Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment
- PMID: 15142273
- PMCID: PMC416448
- DOI: 10.1186/ar1173
Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment
Abstract
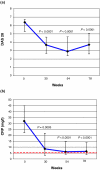
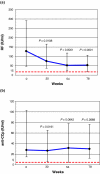
The aim of the present study was to investigate the effect of long-term infliximab treatment on various autoantibodies in patients with rheumatoid arthritis. Serum samples from 30 consecutive patients, who were prospectively followed during infliximab and methotrexate therapy for refractory rheumatoid arthritis, were tested at baseline and after 30, 54 and 78 weeks. At these points, median values of the Disease Activity Score were 6.38 (interquartile range 5.30-6.75), 3.69 (2.67-4.62), 2.9 (2.39-4.65) and 3.71 (2.62-5.06), respectively. Various autoantibodies were assessed by standard indirect immunofluorescence and/or ELISA. Initially, 50% of patients were positive for antinuclear antibodies, and this figure increased to 80% after 78 weeks (P = 0.029). A less marked, similar increase was found for IgG and IgM anticardiolipin antibody titre, whereas the frequency of anti-double-stranded DNA antibodies (by ELISA) exhibited a transient rise (up to 16.7%) at 54 weeks and dropped to 0% at 78 weeks. Antibodies to proteinase-3 and myeloperoxidase were not detected. The proportion of patients who were positive for rheumatoid factor (RF) was similar at baseline and at 78 weeks (87% and 80%, respectively). However, the median RF titre exhibited a progressive reduction from 128 IU/ml (interquartile range 47-290 IU/ml) to 53 IU/ml (18-106 IU/ml). Anti-cyclic citrullinated peptide (CCP) antibodies were found in 83% of patients before therapy; anti-CCP antibody titre significantly decreased at 30 weeks but returned to baseline thereafter. In conclusion, the presence of anti-double-stranded DNA antibodies is a transient phenomenon, despite a stable increase in antinuclear and anticardiolipin antibodies. Also, the evolution of RF titres and that of anti-CCP antibody titres differed during long-term infliximab therapy.
Figures

References
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden Jr, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN, Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
-
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Cannon GW, Spencer-Green G, Finck BK. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–1450. doi: 10.1002/art.10308. - DOI - PubMed
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harrimann G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumor necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
-
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, Ruderman EM, Horwitz DA, Arkefeld DG, Garrison L, Burge DJ, Blosch CM, Lange ML, Mc Donnell ND, Weinblatt ME. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486. - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199803)41:3<565::AID-ART28>3.3.CO;2-#. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

