A direct binding site for Grb2 contributes to transformation and leukemogenesis by the Tel-Abl (ETV6-Abl) tyrosine kinase
- PMID: 15143164
- PMCID: PMC416425
- DOI: 10.1128/MCB.24.11.4685-4695.2004
A direct binding site for Grb2 contributes to transformation and leukemogenesis by the Tel-Abl (ETV6-Abl) tyrosine kinase
Abstract
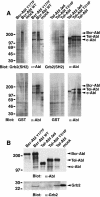
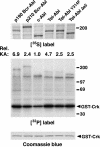
A direct binding site for the Grb2 adapter protein is required for the induction of fatal chronic myeloid leukemia (CML)-like disease in mice by Bcr-Abl. Here, we demonstrate direct binding of Grb2 to the Tel-Abl (ETV6-Abl) fusion protein, the product of complex (9;12) chromosomal translocations in human leukemia, via tyrosine 314 encoded by TEL exon 5. A Tel-Abl point mutant (Y314F) and a splice variant without TEL exon 5 sequences (Deltae5) lacked Grb2 interaction and exhibited decreased binding and phosphorylation of the scaffolding protein Gab2 and impaired activation of phosphatidylinositol 3-kinase, Akt, and extracellular signal-regulated kinase/mitogen-activated protein kinase in hematopoietic cells. Tel-Abl Y314F and Deltae5 were unable to transform fibroblasts to anchorage-independent growth and were defective for B-lymphoid transformation in vitro and lymphoid leukemogenesis in vivo. Previously, we demonstrated that full-length Tel-Abl induced two distinct myeloproliferative diseases in mice: CML-like leukemia similar to that induced by Bcr-Abl and a novel syndrome of small-bowel myeloid infiltration endotoxemia and hepatic and renal failure. Lack of the Grb2 binding site had no effect on development of small bowel syndrome but significantly attenuated the induction of CML-like disease by Tel-Abl. These results suggest that direct binding of Grb2 is a common mechanism contributing to leukemogenesis by oncogenic Abl fusion proteins.
Figures






Similar articles
-
The Grb2 binding site is required for the induction of chronic myeloid leukemia-like disease in mice by the Bcr/Abl tyrosine kinase.Blood. 2000 Jul 15;96(2):664-70. Blood. 2000. PMID: 10887132
-
Critical role for Gab2 in transformation by BCR/ABL.Cancer Cell. 2002 Jun;1(5):479-92. doi: 10.1016/s1535-6108(02)00074-0. Cancer Cell. 2002. PMID: 12124177
-
The Tel-Abl (ETV6-Abl) tyrosine kinase, product of complex (9;12) translocations in human leukemia, induces distinct myeloproliferative disease in mice.Blood. 2002 Jun 15;99(12):4568-77. doi: 10.1182/blood-2001-12-0244. Blood. 2002. PMID: 12036890
-
ETV6: a versatile player in leukemogenesis.Semin Cancer Biol. 2005 Jun;15(3):162-74. doi: 10.1016/j.semcancer.2005.01.008. Semin Cancer Biol. 2005. PMID: 15826831 Review.
-
Molecular pathogenesis of chronic myeloid leukemia: implications for new therapeutic strategies.Ann Hematol. 1999 Feb;78(2):49-64. doi: 10.1007/s002770050473. Ann Hematol. 1999. PMID: 10089019 Review.
Cited by
-
A Case of Chronic Myeloid Leukemia With Rare Variant ETV6/ABL1 Rearrangement.Ann Lab Med. 2017 Jan;37(1):77-80. doi: 10.3343/alm.2017.37.1.77. Ann Lab Med. 2017. PMID: 27834072 Free PMC article. No abstract available.
-
Exploitation of the fibrinolytic system by B-cell acute lymphoblastic leukemia and its therapeutic targeting.Nat Commun. 2024 Nov 20;15(1):10059. doi: 10.1038/s41467-024-54361-4. Nat Commun. 2024. PMID: 39567540 Free PMC article.
-
EML1-ABL1 Is Activated by Coiled-Coil-Mediated Oligomerization and Induces T-Cell Acute Lymphoblastic Leukemia or Myeloproliferative Disease in a Mouse Bone Marrow Transplant Model.Hemasphere. 2018 Mar 6;2(2):e32. doi: 10.1097/HS9.0000000000000032. eCollection 2018 Mar-Apr. Hemasphere. 2018. PMID: 31723760 Free PMC article.
-
The Diverse Roles of ETV6 Alterations in B-Lymphoblastic Leukemia and Other Hematopoietic Cancers.Adv Exp Med Biol. 2024;1459:291-320. doi: 10.1007/978-3-031-62731-6_13. Adv Exp Med Biol. 2024. PMID: 39017849 Review.
-
The role of RAS effectors in BCR/ABL induced chronic myelogenous leukemia.Front Med. 2013 Dec;7(4):452-61. doi: 10.1007/s11684-013-0304-0. Epub 2013 Nov 21. Front Med. 2013. PMID: 24264166
References
-
- Andreasson, P., B. Johansson, M. Carlsson, I. Jarlsfelt, T. Fioretos, F. Mitelman, and M. Hoglund. 1997. BCR/ABL-negative chronic myeloid leukemia with ETV6/ABL fusion. Genes Chromosomes Cancer 20:299-304. - PubMed
-
- Brasher, B. B., S. Roumiantsev, and R. A. Van Etten. 2001. Mutational analysis of the regulatory function of the c-Abl Src homology 3 domain. Oncogene 20:7744-7752. - PubMed
-
- Brunel, V., D. Sainty, N. Carbuccia, M. Mozzicolacci, F. Fernandez, J. Simonetti, J. Gabert, P. Dubreuil, M. Lafage-Pochitaloff, and F. Birg. 1996. A TEL/ABL fusion gene on chromosome 12p13 in a case of Ph−, BCR− atypical CML. Leukemia 10:2003.
-
- Carroll, M., S. Ohno-Jones, S. Tamura, E. Buchdunger, J. Zimmermann, N. B. Lydon, D. G. Gilliland, and B. J. Druker. 1997. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood 90:4947-4952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
