Disruption of CCTbeta2 expression leads to gonadal dysfunction
- PMID: 15143167
- PMCID: PMC416414
- DOI: 10.1128/MCB.24.11.4720-4733.2004
Disruption of CCTbeta2 expression leads to gonadal dysfunction
Abstract
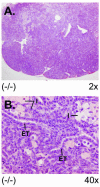
There are two mammalian genes that encode isoforms of CTP:phosphocholine cytidylyltransferase (CCT), a key rate-controlling step in membrane phospholipid biogenesis. Quantitative determination of the CCT transcripts reveals that CCTalpha is ubiquitously expressed and is found at the highest levels in the testis and lung, with lower levels in the liver and ovary. CCTbeta2 is a very minor isoform in most tissues but is significantly expressed in the brain, lung, and gonads. CCTbeta3 is the third isoform recently discovered in mice and is expressed in the same tissues as CCTbeta2, with its highest level in testes. We investigated the role(s) of CCTbeta2 by generating knockout mice. The brains and lungs of mice lacking CCTbeta2 expression did not exhibit any overt defects. On the other hand, a large percentage of the CCTbeta2(-/-) females were sterile and their ovaries exhibited defective ovarian follicle development. The proportion of female CCTbeta2(-/-) mice with defective ovaries increased as the animals aged. The rare litters born from CCTbeta2(-/-) x CCTbeta2(-/0) matings had the normal number of pups. The abnormal ovarian histopathology was characterized by disorganization of the tissue in young adult mice and absence of follicles and ova in older mice, along with interstitial stromal cell hyperplasia which culminated in the emergence of tubulostromal ovarian tumors by 16 months of age. Grossly defective CCTbeta2(-/-) ovaries were associated with high follicle-stimulating (FSH) and luteinizing (LH) hormone levels. Male CCTbeta2(-/0) mice exhibited progressive multifocal testicular degeneration and reduced fertility but had normal FSH and LH levels. Thus, the most notable phenotype of CCTbeta2 knockout mice was gonad degeneration and reproductive deficiency. The results indicate that although CCTbeta2 is expressed at very low levels compared to the alpha-isoform, loss of CCTbeta2 expression causes a breakdown in the gonadal response to hormonal stimulation.
Figures

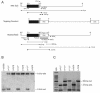
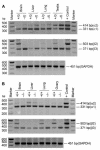

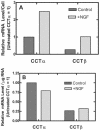
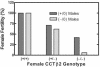




Similar articles
-
Characterization of the murine CTP:phosphocholine cytidylyltransferase beta gene promoter.Biochim Biophys Acta. 2008 May;1781(5):254-62. doi: 10.1016/j.bbalip.2008.03.006. Epub 2008 Apr 1. Biochim Biophys Acta. 2008. PMID: 18423385
-
Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase alpha gene (Pcyt1a).Mol Cell Biol. 2005 Apr;25(8):3357-63. doi: 10.1128/MCB.25.8.3357-3363.2005. Mol Cell Biol. 2005. PMID: 15798219 Free PMC article.
-
Probucol therapy overcomes the reproductive defect in CTP: phosphocholine cytidylyltransferase beta2 knockout mice.Biochim Biophys Acta. 2007 Jul;1771(7):845-52. doi: 10.1016/j.bbalip.2007.04.015. Epub 2007 May 10. Biochim Biophys Acta. 2007. PMID: 17560827 Free PMC article.
-
Chronic hypersecretion of luteinizing hormone in transgenic mice disrupts both ovarian and pituitary function, with some effects modified by the genetic background.Recent Prog Horm Res. 2000;55:69-89; discussion 89-91. Recent Prog Horm Res. 2000. PMID: 11036934 Review.
-
Functional analysis of LHbeta knockout mice.Mol Cell Endocrinol. 2007 Apr 15;269(1-2):81-4. doi: 10.1016/j.mce.2006.10.020. Epub 2007 Feb 12. Mol Cell Endocrinol. 2007. PMID: 17350160 Review.
Cited by
-
Phosphatidylcholine as a metabolic cue for determining B cell fate and function.Cell Immunol. 2016 Dec;310:78-88. doi: 10.1016/j.cellimm.2016.08.002. Epub 2016 Aug 3. Cell Immunol. 2016. PMID: 27502364 Free PMC article.
-
The mammalian ovary from genesis to revelation.Endocr Rev. 2009 Oct;30(6):624-712. doi: 10.1210/er.2009-0012. Epub 2009 Sep 23. Endocr Rev. 2009. PMID: 19776209 Free PMC article. Review.
-
Differential dephosphorylation of CTP:phosphocholine cytidylyltransferase upon translocation to nuclear membranes and lipid droplets.Mol Biol Cell. 2020 May 1;31(10):1047-1059. doi: 10.1091/mbc.E20-01-0014. Epub 2020 Mar 18. Mol Biol Cell. 2020. PMID: 32186954 Free PMC article.
-
PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress.Dev Cell. 2018 May 21;45(4):481-495.e8. doi: 10.1016/j.devcel.2018.04.012. Epub 2018 May 10. Dev Cell. 2018. PMID: 29754800 Free PMC article.
-
ras-Induced up-regulation of CTP:phosphocholine cytidylyltransferase α contributes to malignant transformation of intestinal epithelial cells.J Biol Chem. 2013 Jan 4;288(1):633-43. doi: 10.1074/jbc.M112.347682. Epub 2012 Nov 15. J Biol Chem. 2013. PMID: 23155050 Free PMC article.
References
-
- Arnold, R. S., and R. B. Cornell. 1996. Lipid regulation of CTP:phosphocholine cytidylyltransferase: electrostatic, hydrophobic, and synergistic interactions of anionic phospholipids and diacylglycerol. Biochemistry 35:9917-9924. - PubMed
-
- Arnold, R. S., A. A. DePaoli-Roach, and R. B. Cornell. 1997. Binding of CTP:phosphocholine cytidylyltransferase to lipid vesicles: diacylglycerol and enzyme dephosphorylation increase the affinity for negatively charged membranes. Biochemistry 36:6149-6156. - PubMed
-
- Baburina, I., and S. Jackowski. 1999. Cellular responses to excess phospholipid. J. Biol. Chem. 274:9400-9408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
