Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin
- PMID: 15143170
- PMCID: PMC416421
- DOI: 10.1128/MCB.24.11.4757-4768.2004
Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin
Abstract
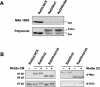
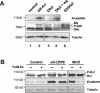
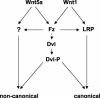
Wnt glycoproteins play essential roles in the development of metazoan organisms. Many Wnt proteins, such as Wnt1, activate the well-conserved canonical Wnt signaling pathway, which results in accumulation of beta-catenin in the cytosol and nucleus. Other Wnts, such as Wnt5a, activate signaling mechanisms which do not involve beta-catenin and are less well characterized. Dishevelled (Dvl) is a key component of Wnt/beta-catenin signaling and becomes phosphorylated upon activation of this pathway. In addition to Wnt1, we show that several Wnt proteins, including Wnt5a, trigger phosphorylation of mammalian Dvl proteins and that this occurs within 20 to 30 min. Unlike the effects of Wnt1, phosphorylation of Dvl in response to Wnt5a is not concomitant with beta-catenin stabilization, indicating that Dvl phosphorylation is not sufficient to activate canonical Wnt/beta-catenin signaling. Moreover, neither Dickkopf1, which inhibits Wnt/beta-catenin signaling by binding the Wnt coreceptors LRP5 and -6, nor dominant-negative LRP5/6 constructs could block Wnt-mediated Dvl phosphorylation. We conclude that Wnt-induced phosphorylation of Dvl is independent of LRP5/6 receptors and that canonical Wnts can elicit both LRP-dependent (to beta-catenin) and LRP-independent (to Dvl) signals. Our data also present Dvl phosphorylation as a general biochemical assay for Wnt protein function, including those Wnts that do not activate the Wnt/beta-catenin pathway.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
