The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins
- PMID: 15143178
- PMCID: PMC416415
- DOI: 10.1128/MCB.24.11.4848-4857.2004
The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins
Abstract
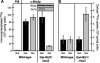
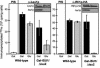
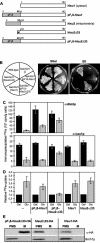
Iron-sulfur (Fe/S) proteins are located in mitochondria, cytosol, and nucleus. Mitochondrial Fe/S proteins are matured by the iron-sulfur cluster (ISC) assembly machinery. Little is known about the formation of Fe/S proteins in the cytosol and nucleus. A function of mitochondria in cytosolic Fe/S protein maturation has been noted, but small amounts of some ISC components have been detected outside mitochondria. Here, we studied the highly conserved yeast proteins Isu1p and Isu2p, which provide a scaffold for Fe/S cluster synthesis. We asked whether the Isu proteins are needed for biosynthesis of cytosolic Fe/S clusters and in which subcellular compartment the Isu proteins are required. The Isu proteins were found to be essential for de novo biosynthesis of both mitochondrial and cytosolic Fe/S proteins. Several lines of evidence indicate that Isu1p and Isu2p have to be located inside mitochondria in order to perform their function in cytosolic Fe/S protein maturation. We were unable to mislocalize Isu1p to the cytosol due to the presence of multiple, independent mitochondrial targeting signals in this protein. Further, the bacterial homologue IscU and the human Isu proteins (partially) complemented the defects of yeast Isu protein-depleted cells in growth rate, Fe/S protein biogenesis, and iron homeostasis, yet only after targeting to mitochondria. Together, our data suggest that the Isu proteins need to be localized in mitochondria to fulfill their functional requirement in Fe/S protein maturation in the cytosol.
Figures



Similar articles
-
Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p.EMBO J. 2003 Sep 15;22(18):4815-25. doi: 10.1093/emboj/cdg446. EMBO J. 2003. PMID: 12970193 Free PMC article.
-
Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins.EMBO J. 2006 Jan 11;25(1):184-95. doi: 10.1038/sj.emboj.7600906. Epub 2005 Dec 8. EMBO J. 2006. PMID: 16341089 Free PMC article.
-
Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes.Biochim Biophys Acta. 2006 Jul;1763(7):652-67. doi: 10.1016/j.bbamcr.2006.05.011. Epub 2006 May 23. Biochim Biophys Acta. 2006. PMID: 16843540 Review.
-
The Hsp70 chaperone Ssq1p is dispensable for iron-sulfur cluster formation on the scaffold protein Isu1p.J Biol Chem. 2006 Mar 24;281(12):7801-8. doi: 10.1074/jbc.M513301200. Epub 2006 Jan 23. J Biol Chem. 2006. PMID: 16431909
-
Mitochondrial iron-sulfur protein biogenesis and human disease.Biochimie. 2014 May;100:61-77. doi: 10.1016/j.biochi.2014.01.010. Epub 2014 Jan 23. Biochimie. 2014. PMID: 24462711 Review.
Cited by
-
The eukaryotic P loop NTPase Nbp35: an essential component of the cytosolic and nuclear iron-sulfur protein assembly machinery.Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3266-71. doi: 10.1073/pnas.0406447102. Epub 2005 Feb 22. Proc Natl Acad Sci U S A. 2005. PMID: 15728363 Free PMC article.
-
Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway.Mol Cell Biol. 2007 Apr;27(8):2841-7. doi: 10.1128/MCB.01321-06. Epub 2007 Feb 5. Mol Cell Biol. 2007. PMID: 17283054 Free PMC article.
-
Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes.Mol Cell Biol. 2008 Mar;28(5):1851-61. doi: 10.1128/MCB.01963-07. Epub 2007 Dec 17. Mol Cell Biol. 2008. PMID: 18086897 Free PMC article.
-
Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron-sulfur cluster scaffold Isu protein is mutually exclusive.J Biol Chem. 2013 Oct 4;288(40):29134-42. doi: 10.1074/jbc.M113.503524. Epub 2013 Aug 14. J Biol Chem. 2013. PMID: 23946486 Free PMC article.
-
Function and biogenesis of iron-sulphur proteins.Nature. 2009 Aug 13;460(7257):831-8. doi: 10.1038/nature08301. Nature. 2009. PMID: 19675643 Review.
References
-
- Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. - PubMed
-
- Beinert, H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Bioinorg. Chem. 5:2-15. - PubMed
-
- Beinert, H., R. H. Holm, and E. Münck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. - PubMed
-
- Bekri, S., G. Kispal, H. Lange, E. Fitzsimons, J. Tolmie, R. Lill, and D. F. Bishop. 2000. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia (XLSA/A) with disruption of cytosolic iron-sulfur protein maturation. Blood 96:3256-3264. - PubMed
-
- Bouton, C., M. J. Chauveau, S. Lazereg, and J. C. Drapier. 2002. Recycling of RNA binding iron regulatory protein 1 into an aconitase after nitric oxide removal depends on mitochondrial ATP. J. Biol. Chem. 277:31220-31227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
