Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding
- PMID: 15143181
- PMCID: PMC416409
- DOI: 10.1128/MCB.24.11.4880-4894.2004
Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding
Abstract
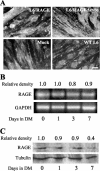
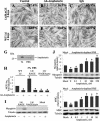
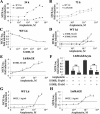
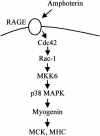
The receptor for advanced glycation end products (RAGE), a multiligand receptor of the immunoglobulin superfamily, has been implicated in the inflammatory response, diabetic angiopathy and neuropathy, neurodegeneration, cell migration, tumor growth, neuroprotection, and neuronal differentiation. We show here that (i) RAGE is expressed in skeletal muscle tissue and its expression is developmentally regulated and (ii) RAGE engagement by amphoterin (HMGB1), a RAGE ligand, in rat L6 myoblasts results in stimulation of myogenic differentiation via activation of p38 mitogen-activated protein kinase (MAPK), up-regulation of myogenin and myosin heavy chain expression, and induction of muscle creatine kinase. No such effects were detected in myoblasts transfected with a RAGE mutant lacking the transducing domain or myoblasts transfected with a constitutively inactive form of the p38 MAPK upstream kinase, MAPK kinase 6, Cdc42, or Rac-1. Moreover, amphoterin counteracted the antimyogenic activity of the Ca(2+)-modulated protein S100B, which was reported to inhibit myogenic differentiation via inactivation of p38 MAPK, and basic fibroblast growth factor (bFGF), a known inhibitor of myogenic differentiation, in a manner that was inversely related to the S100B or bFGF concentration and directly related to the extent of RAGE expression. These data suggest that RAGE and amphoterin might play an important role in myogenesis, accelerating myogenic differentiation via Cdc42-Rac-1-MAPK kinase 6-p38 MAPK.
Figures




Similar articles
-
The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo.J Biol Chem. 2006 Mar 24;281(12):8242-53. doi: 10.1074/jbc.M509436200. Epub 2006 Jan 9. J Biol Chem. 2006. PMID: 16407300
-
S100B inhibits myogenic differentiation and myotube formation in a RAGE-independent manner.Mol Cell Biol. 2003 Jul;23(14):4870-81. doi: 10.1128/MCB.23.14.4870-4881.2003. Mol Cell Biol. 2003. PMID: 12832473 Free PMC article.
-
RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth.Am J Pathol. 2007 Sep;171(3):947-61. doi: 10.2353/ajpath.2007.070049. Epub 2007 Jul 19. Am J Pathol. 2007. PMID: 17640970 Free PMC article.
-
S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain.Biochim Biophys Acta. 2004 Dec 6;1742(1-3):169-77. doi: 10.1016/j.bbamcr.2004.09.008. Biochim Biophys Acta. 2004. PMID: 15590067 Review.
-
RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins.Curr Mol Med. 2007 Dec;7(8):711-24. doi: 10.2174/156652407783220688. Curr Mol Med. 2007. PMID: 18331229 Review.
Cited by
-
S100B protein in tissue development, repair and regeneration.World J Biol Chem. 2013 Feb 26;4(1):1-12. doi: 10.4331/wjbc.v4.i1.1. World J Biol Chem. 2013. PMID: 23580916 Free PMC article.
-
Engineering spatial control of multiple differentiation fates within a stem cell population.Biomaterials. 2011 May;32(13):3413-22. doi: 10.1016/j.biomaterials.2011.01.036. Epub 2011 Feb 12. Biomaterials. 2011. PMID: 21316755 Free PMC article.
-
Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE.J Matern Fetal Neonatal Med. 2012 Jun;25(6):558-67. doi: 10.3109/14767058.2011.599083. J Matern Fetal Neonatal Med. 2012. PMID: 22578261 Free PMC article.
-
HuR and miR-1192 regulate myogenesis by modulating the translation of HMGB1 mRNA.Nat Commun. 2013;4:2388. doi: 10.1038/ncomms3388. Nat Commun. 2013. PMID: 24005720 Free PMC article.
-
Genetically-determined hyperfunction of the S100B/RAGE axis is a risk factor for aspergillosis in stem cell transplant recipients.PLoS One. 2011;6(11):e27962. doi: 10.1371/journal.pone.0027962. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22114731 Free PMC article.
References
-
- Arnold, H. H., and B. Winter. 1998. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 8:539-544. - PubMed
-
- Arumugam, T., D. M. Simeone, A. M. Schmidt, and C. D. Logsdon. 2004. S100P stimulates cell proliferation and survival via RAGE. J. Biol. Chem. 279:5059-5065. (First published 14 November 2003; 10.1074/jbc.M310124200.) - PubMed
-
- Bennett, A. M., and N. K. Tonks. 1997. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288-1291. - PubMed
-
- Bergstrom, D. A., B. H. Penn, A. Strand, R. L. Perry, M. A. Rudnicki, and S. J. Tapscott. 2002. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9:587-600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
