Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action
- PMID: 15143191
- PMCID: PMC416420
- DOI: 10.1128/MCB.24.11.5005-5015.2004
Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action
Abstract
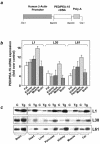
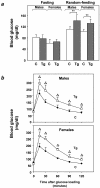
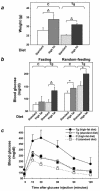
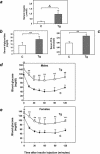
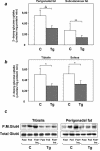
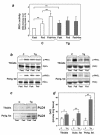
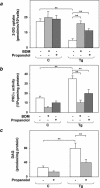
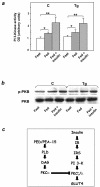
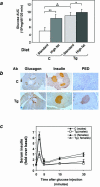
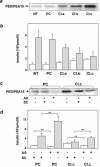
Overexpression of the ped/pea-15 gene is a common feature of type 2 diabetes. In the present work, we show that transgenic mice ubiquitously overexpressing ped/pea-15 exhibited mildly elevated random-fed blood glucose levels and decreased glucose tolerance. Treatment with a 60% fat diet led ped/pea-15 transgenic mice to develop diabetes. Consistent with insulin resistance in these mice, insulin administration reduced glucose levels by only 35% after 45 min, compared to 70% in control mice. In vivo, insulin-stimulated glucose uptake was decreased by almost 50% in fat and muscle tissues of the ped/pea-15 transgenic mice, accompanied by protein kinase Calpha activation and block of insulin induction of protein kinase Czeta. These changes persisted in isolated adipocytes from the transgenic mice and were rescued by the protein kinase C inhibitor bisindolylmaleimide. In addition to insulin resistance, ped/pea-15 transgenic mice showed a 70% reduction in insulin response to glucose loading. Stable overexpression of ped/pea-15 in the glucose-responsive MIN6 beta-cell line also caused protein kinase Calpha activation and a marked decline in glucose-stimulated insulin secretion. Antisense block of endogenous ped/pea-15 increased glucose sensitivity by 2.5-fold in these cells. Thus, in vivo, overexpression of ped/pea-15 may lead to diabetes by impairing insulin secretion in addition to insulin action.
Figures

Similar articles
-
Adenoviral gene transfer of PLD1-D4 enhances insulin sensitivity in mice by disrupting phospholipase D1 interaction with PED/PEA-15.PLoS One. 2013 Apr 9;8(4):e60555. doi: 10.1371/journal.pone.0060555. Print 2013. PLoS One. 2013. PMID: 23585839 Free PMC article.
-
PED/PEA-15 regulates glucose-induced insulin secretion by restraining potassium channel expression in pancreatic beta-cells.Diabetes. 2007 Mar;56(3):622-33. doi: 10.2337/db06-1260. Diabetes. 2007. PMID: 17327429
-
Peroxisome proliferator-activated receptor-γ activation enhances insulin-stimulated glucose disposal by reducing ped/pea-15 gene expression in skeletal muscle cells: evidence for involvement of activator protein-1.J Biol Chem. 2012 Dec 14;287(51):42951-61. doi: 10.1074/jbc.M112.406637. Epub 2012 Oct 26. J Biol Chem. 2012. PMID: 23105093 Free PMC article.
-
Frontiers: PED/PEA-15, a multifunctional protein controlling cell survival and glucose metabolism.Am J Physiol Endocrinol Metab. 2009 Sep;297(3):E592-601. doi: 10.1152/ajpendo.00228.2009. Epub 2009 Jun 16. Am J Physiol Endocrinol Metab. 2009. PMID: 19531639 Review.
-
Targetting PED/PEA-15 for diabetes treatment.Expert Opin Ther Targets. 2017 Jun;21(6):571-581. doi: 10.1080/14728222.2017.1317749. Epub 2017 Apr 21. Expert Opin Ther Targets. 2017. PMID: 28395542 Review.
Cited by
-
Mechanisms of Diabetic Nephropathy Not Mediated by Hyperglycemia.J Clin Med. 2023 Oct 30;12(21):6848. doi: 10.3390/jcm12216848. J Clin Med. 2023. PMID: 37959313 Free PMC article. Review.
-
Tyrosine phosphorylation of phosphoinositide-dependent kinase 1 by the insulin receptor is necessary for insulin metabolic signaling.Mol Cell Biol. 2005 Dec;25(24):10803-14. doi: 10.1128/MCB.25.24.10803-10814.2005. Mol Cell Biol. 2005. PMID: 16314505 Free PMC article.
-
Prep1 deficiency induces protection from diabetes and increased insulin sensitivity through a p160-mediated mechanism.Mol Cell Biol. 2008 Sep;28(18):5634-45. doi: 10.1128/MCB.00117-08. Epub 2008 Jul 21. Mol Cell Biol. 2008. PMID: 18644868 Free PMC article.
-
Sodium-glucose cotransporter-2 inhibitor alleviated atrial remodeling in STZ-induced diabetic rats by targeting TLR4 pathway.Front Cardiovasc Med. 2022 Sep 6;9:908037. doi: 10.3389/fcvm.2022.908037. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36148071 Free PMC article.
-
PED/PEA-15 inhibits hydrogen peroxide-induced apoptosis in Ins-1E pancreatic beta-cells via PLD-1.PLoS One. 2014 Dec 9;9(12):e113655. doi: 10.1371/journal.pone.0113655. eCollection 2014. PLoS One. 2014. PMID: 25489735 Free PMC article.
References
-
- Arner, P. 2002. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab. Res. Rev. 18(Suppl. 2):S5-S9. - PubMed
-
- Bell, G. I., and K. S. Polonsky. 2001. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414:788-791. - PubMed
-
- Boden, G., and G. I. Shulman. 2002. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 32(Suppl. 3):14-23. - PubMed
-
- Caruso, M., C. Miele, A. Oliva, G. Condorelli, F. Oriente, G. Riccardi, B. Capaldo, F. Fiory, D. Accili, P. Formisano, and F. Beguinot. 2000. The IR1152 mutant insulin receptor selectively impairs insulin action in skeletal muscle but not in liver. Diabetes 49:1194-1202. - PubMed
-
- Condorelli, G., A. Trencia, G. Vigliotta, A. Perfetti, U. Goglia, A. Cassese, A. M. Musti, C. Miele, S. Santopietro, P. Formisano, and F. Beguinot. 2002. Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J. Biol. Chem. 277:11013-11018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
