Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis
- PMID: 15144852
- PMCID: PMC7125733
- DOI: 10.1016/j.expneurol.2004.01.028
Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis
Abstract
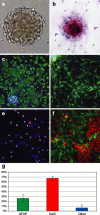
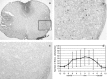
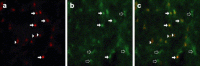
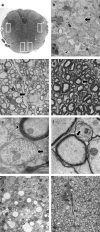
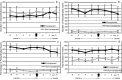
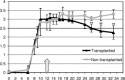
The behavior and myelinogenic properties of glial cells have been well documented following transplantation into regions of focal experimental demyelination in animal models. However, the ability of glial cell preparations to remyelinate in such models does not necessarily indicate that their transplantation into demyelinated lesions in clinical disease will be successful. One of the precluding factors in this regard is a greater understanding of the environmental conditions that will support transplant-mediated remyelination. In this study, we determined whether the complex and reactive CNS environment of the mouse hepatitis virus (MHV) model of multiple sclerosis (MS) could support transplant-mediated remyelination. Striatal neural precursors derived from postnatal day 1 mice were committed to a glial cell lineage and labeled. Immunohistochemical staining indicated that this population generated >93% glial cells following differentiation in vitro. Transplantation of glial-committed progenitor cells into the T8 spinal cord of MHV-infected mice demonstrating complete hindlimb paralysis resulted in migration of cells up to 12 mm from the implantation site and remyelination of up to 67% of axons. Transplanted-remyelinated animals contained approximately 2x the number of axons within sampled regions of the ventral and lateral columns as compared to non-transplanted animals, suggesting that remyelination is associated with axonal sparing. Furthermore, transplantation resulted in behavioral improvement. This study demonstrates for the first time that transplant-mediated remyelination is possible in the pathogenic environment of the MHV demyelination model and that it is associated with locomotor improvement.
Figures
Similar articles
-
Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response.Exp Neurol. 2006 Feb;197(2):420-9. doi: 10.1016/j.expneurol.2005.10.016. Epub 2005 Nov 17. Exp Neurol. 2006. PMID: 16297915 Free PMC article.
-
Two-photon imaging of remyelination of spinal cord axons by engrafted neural precursor cells in a viral model of multiple sclerosis.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):E2349-55. doi: 10.1073/pnas.1406658111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843159 Free PMC article.
-
Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination.Glia. 2017 Aug;65(8):1350-1360. doi: 10.1002/glia.23167. Epub 2017 May 31. Glia. 2017. PMID: 28560740 Free PMC article.
-
Glial precursor cell transplantation therapy for neurotrauma and multiple sclerosis.Prog Histochem Cytochem. 2008;43(3):123-76. doi: 10.1016/j.proghi.2008.04.001. Epub 2008 Jun 13. Prog Histochem Cytochem. 2008. PMID: 18706353 Review.
-
Transplantation options for therapeutic central nervous system remyelination.Cell Transplant. 2000 Mar-Apr;9(2):289-94. doi: 10.1177/096368970000900214. Cell Transplant. 2000. PMID: 10811401 Review.
Cited by
-
CXCR2 signaling protects oligodendrocytes and restricts demyelination in a mouse model of viral-induced demyelination.PLoS One. 2010 Jun 28;5(6):e11340. doi: 10.1371/journal.pone.0011340. PLoS One. 2010. PMID: 20596532 Free PMC article.
-
Cell replacement therapies to promote remyelination in a viral model of demyelination.J Neuroimmunol. 2010 Jul 27;224(1-2):101-7. doi: 10.1016/j.jneuroim.2010.05.013. Epub 2010 Jun 2. J Neuroimmunol. 2010. PMID: 20627412 Free PMC article. Review.
-
Cell replacement therapy with stem cells in multiple sclerosis, a systematic review.Hum Cell. 2024 Jan;37(1):9-53. doi: 10.1007/s13577-023-01006-1. Epub 2023 Nov 21. Hum Cell. 2024. PMID: 37985645 Free PMC article.
-
Quetiapine Attenuates Schizophrenia-Like Behaviors and Demyelination in a MK-801-Induced Mouse Model of Schizophrenia.Front Psychiatry. 2020 Aug 19;11:843. doi: 10.3389/fpsyt.2020.00843. eCollection 2020. Front Psychiatry. 2020. PMID: 32973585 Free PMC article.
-
Inflammation induced by infection potentiates tau pathological features in transgenic mice.Am J Pathol. 2011 Jun;178(6):2811-22. doi: 10.1016/j.ajpath.2011.02.012. Epub 2011 Apr 30. Am J Pathol. 2011. PMID: 21531375 Free PMC article.
References
-
- Arnold D.L., Riess G.T., Matthews P.M., Francis G.S., Collins D.L., Wolfson C., Antel J.P. Use of proton magnetic resonance spectroscopy for monitoring disease progression in multiple sclerosis. Ann. Neurol. 1994;36:76–82. - PubMed
-
- Ben-Hur T., Einstein O., Mizrachi-Kol R., Ben-Menachem O., Reinhartz E., Karussis D., Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. - PubMed
-
- Bitsch A., Schuchardt J., Bunkowski S., Kuhlmann T., Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. - PubMed
-
- Bjartmar C., Trapp B.D. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr. Opin. Neurol. 2001;14:271–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

