Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus
- PMID: 15146040
- PMCID: PMC1664907
- DOI: 10.1113/jphysiol.2004.066654
Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus
Abstract
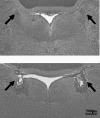
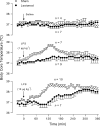
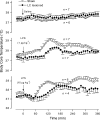
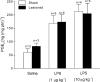
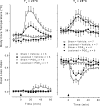
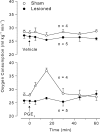
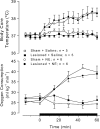
It is known that brain noradrenaline (norepinephrine) mediates fever, but the neuronal group involved is unknown. We studied the role of the major noradrenergic nucleus, the locus coeruleus (LC), in lipopolysaccharide (LPS)-induced fever. Male Wistar rats had their LC completely ablated electrolytically or their catecholaminergic LC neurones selectively lesioned by microinjection of 6-hydroxydopamine; the controls were sham-operated. Both lesions resulted in a marked attenuation of LPS (1 or 10 microg kg(-1), i.v.) fever at a subneutral (23 degrees C) ambient temperature (Ta). Because electrolytic and chemical lesions produced similar effects, the role of the LC in fever was further investigated using electrolytic lesions only. The levels of prostaglandin (PG) E2, the terminal mediator of fever, were equally raised in the anteroventral third ventricular region of LC-lesioned and sham-operated rats during the course of LPS fever, indicating that LC neurones are not involved in febrigenic signalling to the brain. To investigate the potential involvement of the LC in an efferent thermoregulatory neuronal pathway, the thermoregulatory response to PGE(2) (25 ng, i.c.v.) was studied at a subneutral (23 degrees C, when fever is brought about by thermogenesis) or neutral (28 degrees C, when fever is brought about by tail skin vasoconstriction) Ta. The PGE2-induced increases in metabolic rate (an index of thermogenesis) and fever were attenuated in LC-lesioned rats at 23 degrees C, whereas PGE2-induced skin vasoconstriction and fever normally developed in LC-lesioned rats at 28 degrees C. The LC-lesioned rats had attenuated PGE2 thermogenesis despite the fact that they were fully capable of activating thermogenesis in response to noradrenaline and cold exposure. It is concluded that LC neurones are part of a neuronal network that is specifically activated by PGE2 to increase thermogenesis and produce fever.
Figures


References
-
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, Charléty P, Valentino RJ, Williams JT. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. - PubMed
-
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. Sydney: Academic Press, Inc.; 1995. pp. 183–213.
-
- Bachtell RK, Tsivkovskaia NO, Ryabinin E. Identification of temperature-sensitive neural circuits in mice using c-fos expression mapping. Brain Res. 2003;960:157–164. - PubMed
-
- Berridge CW, Waterhouse BD. The locus coeruleus—noradrenergic system: modulation of behavioral state and state-dependent cognitive processess. Brain Res Rev. 2003;42:33–84. - PubMed
-
- Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997a;12:1–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

