Adenosine decreases both presynaptic calcium currents and neurotransmitter release at the mouse neuromuscular junction
- PMID: 15146054
- PMCID: PMC1664967
- DOI: 10.1113/jphysiol.2004.061457
Adenosine decreases both presynaptic calcium currents and neurotransmitter release at the mouse neuromuscular junction
Abstract
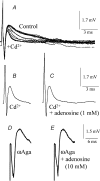
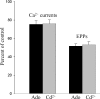
A controversy currently exists as to the mechanism of action by which adenosine, an endogenous mediator of neurotransmitter depression, reduces the evoked release of the neurotransmitter acetylcholine (ACh) at the skeletal neuromuscular junction. Specifically, it is uncertain whether adenosine inhibits ACh release from mammalian motor nerve endings by reducing Ca(2+) calcium entry through voltage-gated calcium channels or, as is the case at amphibian motor nerve endings, by an effect downstream of Ca(2+) entry. In an attempt to address this controversy, the effects of adenosine on membrane ionic currents and neurotransmitter release were studied at neuromuscular junctions in adult mouse phrenic nerve hemidiaphragm preparations. In wild-type mice, adenosine (500 microm-1 mm) reduced prejunctional Ca(2+) currents simultaneously with a reduction in evoked ACh release. In Rab3A knockout mice, which have been shown to have an increased sensitivity to adenosine, the simultaneous reduction in Ca(2+) currents and ACh secretion occurred at significantly lower adenosine concentrations (< or = 50 microM). Measurements of nerve terminal Na(+) and K(+) currents made simultaneously with evoked ACh release demonstrated that the decreases in Ca(2+) currents were not attributable to changes in cation entry through voltage-gated Na(+) or K(+) channels. Furthermore, no effects of adenosine on residual ionic currents were observed when P/Q-type calcium channels were blocked by Cd(2+) or omega-agatoxin-IVA. The results demonstrate that inhibition of evoked neurotransmitter secretion by adenosine is associated with a reduction in Ca(2+) calcium entry through voltage-gated P/Q Ca(2+) channels at the mouse neuromuscular junction. Whilst it may be that adenosine inhibits ACh release by different mechanisms at amphibia and mammalian neuromuscular junctions, it is also possible that the secretory apparatus is more intimately coupled to the Ca(2+) channels in the mouse such that an effect on the secretory machinery is reflected as changes in Ca(2+) currents.
Figures
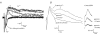
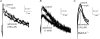


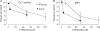
Similar articles
-
Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction.Neuroscience. 2006 Sep 29;142(1):71-85. doi: 10.1016/j.neuroscience.2006.05.062. Epub 2006 Jul 14. Neuroscience. 2006. PMID: 16843602
-
Presynaptic inhibition of spontaneous acetylcholine release induced by adenosine at the mouse neuromuscular junction.Br J Pharmacol. 2004 May;142(1):113-24. doi: 10.1038/sj.bjp.0705656. Epub 2004 Apr 5. Br J Pharmacol. 2004. PMID: 15066904 Free PMC article.
-
Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction.Br J Pharmacol. 2013 Aug;169(8):1810-23. doi: 10.1111/bph.12262. Br J Pharmacol. 2013. PMID: 23731236 Free PMC article.
-
Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions (Review).Mol Membr Biol. 2002 Oct-Dec;19(4):293-300. doi: 10.1080/0968768021000035087. Mol Membr Biol. 2002. PMID: 12512776 Review.
-
Ca2+ channels and synaptic transmission at the adult, neonatal, and P/Q-type deficient neuromuscular junction.Ann N Y Acad Sci. 2003 Sep;998:11-7. doi: 10.1196/annals.1254.003. Ann N Y Acad Sci. 2003. PMID: 14592858 Review.
Cited by
-
Modulation of calcium-dependent and -independent acetylcholine release from motor nerve endings.J Mol Neurosci. 2006;30(1-2):215-8. doi: 10.1385/JMN:30:1:215. J Mol Neurosci. 2006. PMID: 17192679
-
A1 Adenosine Receptor-Mediated Inhibition of Parasympathetic Neuromuscular Transmission in Human and Murine Urinary Bladder.J Pharmacol Exp Ther. 2016 Jan;356(1):116-22. doi: 10.1124/jpet.115.228882. Epub 2015 Nov 3. J Pharmacol Exp Ther. 2016. PMID: 26534943 Free PMC article.
-
Purinergic modulation of synaptic signalling at the neuromuscular junction.Pflugers Arch. 2006 Aug;452(5):608-14. doi: 10.1007/s00424-006-0068-3. Epub 2006 Apr 8. Pflugers Arch. 2006. PMID: 16604367 Review.
-
Purinergic Tuning of the Tripartite Neuromuscular Synapse.Mol Neurobiol. 2023 Jul;60(7):4084-4104. doi: 10.1007/s12035-023-03317-8. Epub 2023 Apr 5. Mol Neurobiol. 2023. PMID: 37016047 Free PMC article. Review.
-
Evidence for constitutively-active adenosine receptors at mammalian motor nerve endings.Eur J Pharmacol. 2012 Jun 15;685(1-3):38-41. doi: 10.1016/j.ejphar.2012.04.008. Epub 2012 Apr 20. Eur J Pharmacol. 2012. PMID: 22542659 Free PMC article.
References
-
- Blackmer T, Larsen EC, Takahashi M, Martin TFJ, Alford S, Hamm HE. G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. - PubMed
-
- Deist M, Repp H, Dreyer F. Sulfonourea-sensitive K channels and their probable role for the membrane potential of mouse motor nerve endings. Pflugers Archs. 1992;42:292–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

