Transmembrane peptides as inhibitors of ErbB receptor signaling
- PMID: 15146055
- PMCID: PMC452597
- DOI: 10.1091/mbc.e03-10-0753
Transmembrane peptides as inhibitors of ErbB receptor signaling
Abstract
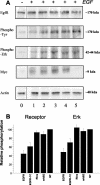
Receptor tyrosine kinases have a single transmembrane (TM) segment that is usually assumed to play a passive role in ligand-induced dimerization and activation of the receptor. However, mutations within some of these receptors, and recent studies with the epidermal growth factor (EGF) and ErbB2 receptors have indicated that interactions between TM domains do contribute to stabilization of ligand-independent and/or ligand-induced receptor dimerization and activation. One consequence of the importance of these interactions is that short hydrophobic peptides corresponding to these domains should act as specific inhibitors. To test this hypothesis, we constructed expression vectors encoding short fusion peptides encompassing native or mutated TM domains of the EGF, ErbB2, and insulin receptors. In human cell lines overexpressing the wild-type EGF receptor or ErbB2, we observed that the peptides are expressed at the cell surface and that they inhibit specifically the autophosphorylation and signaling pathway of their cognate receptor. Identical results were obtained with peptides chemically synthesized. Mechanism of action involves inhibition of dimerization of the receptors as shown by the lack of effects of mutant nondimerizing sequences, completed by density centrifugation and covalent cross-linking experiments. Our findings stress the role of TM domain interactions in ErbB receptor function, and possibly for other single-spanning membrane proteins.
Figures




References
-
- Bargmann, C.I., Hung, M.C., and Weinberg, R.A. (1986). Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 45, 649-657. - PubMed
-
- Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355-365. - PubMed
-
- Burke, C.L., Lemmon, M.A., Coren, B.A., Engelman, D.M., and Stern, D.F. (1997). Dimerization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene 14, 687-696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

