Complete cycles of bloodstream trypanosome RNA editing in vitro
- PMID: 15146075
- PMCID: PMC1370583
- DOI: 10.1261/rna.5157704
Complete cycles of bloodstream trypanosome RNA editing in vitro
Abstract
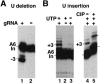
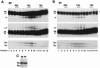
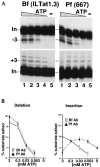
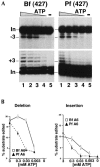
RNA editing in kinetoplastid protists is required for the maturation of mitochondrial pre-mRNAs and occurs by protein-catalyzed cycles of uridylate insertion and deletion. During the complex life cycle of Trypanosoma brucei this process is differentially regulated in the mammalian bloodstream and insect procyclic stages. Complementary guide RNAs (gRNAs) direct editing, but the abundance of these transcripts is not developmentally controlled. The establishment of in vitro systems that recreate efficient RNA editing in bloodstream T. brucei would be valuable for mechanistic studies of regulation. Here we describe a robust in vitro system that reconstitutes full cycles of both U insertion and U deletion in bloodstream trypanosomes, and the first direct comparisons of the in vitro systems for strains of mammalian and insect stages.
Figures
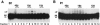
Similar articles
-
Analysis of the Trypanosoma brucei EATRO 164 Bloodstream Guide RNA Transcriptome.PLoS Negl Trop Dis. 2016 Jul 11;10(7):e0004793. doi: 10.1371/journal.pntd.0004793. eCollection 2016 Jul. PLoS Negl Trop Dis. 2016. PMID: 27399202 Free PMC article.
-
Guide RNAs for transcripts with developmentally regulated RNA editing are present in both life cycle stages of Trypanosoma brucei.Mol Cell Biol. 1992 May;12(5):2043-9. doi: 10.1128/mcb.12.5.2043-2049.1992. Mol Cell Biol. 1992. PMID: 1373804 Free PMC article.
-
KREX2 is not essential for either procyclic or bloodstream form Trypanosoma brucei.PLoS One. 2012;7(3):e33405. doi: 10.1371/journal.pone.0033405. Epub 2012 Mar 15. PLoS One. 2012. PMID: 22438925 Free PMC article.
-
Trypanosome RNA editing: the complexity of getting U in and taking U out.Wiley Interdiscip Rev RNA. 2016 Jan-Feb;7(1):33-51. doi: 10.1002/wrna.1313. Epub 2015 Nov 2. Wiley Interdiscip Rev RNA. 2016. PMID: 26522170 Free PMC article. Review.
-
Lexis and Grammar of Mitochondrial RNA Processing in Trypanosomes.Trends Parasitol. 2020 Apr;36(4):337-355. doi: 10.1016/j.pt.2020.01.006. Epub 2020 Feb 28. Trends Parasitol. 2020. PMID: 32191849 Free PMC article. Review.
Cited by
-
RNA editing complex interactions with a site for full-round U deletion in Trypanosoma brucei.RNA. 2006 Jul;12(7):1219-28. doi: 10.1261/rna.2295706. Epub 2006 May 11. RNA. 2006. PMID: 16690999 Free PMC article.
-
RBP16 stimulates trypanosome RNA editing in vitro at an early step in the editing reaction.RNA. 2006 Jul;12(7):1292-303. doi: 10.1261/rna.2331506. Epub 2006 May 11. RNA. 2006. PMID: 16691000 Free PMC article.
-
Guide RNA biogenesis involves a novel RNase III family endoribonuclease in Trypanosoma brucei.RNA. 2011 Oct;17(10):1821-30. doi: 10.1261/rna.2815911. Epub 2011 Aug 2. RNA. 2011. PMID: 21810935 Free PMC article.
-
A Protein Complex Map of Trypanosoma brucei.PLoS Negl Trop Dis. 2016 Mar 18;10(3):e0004533. doi: 10.1371/journal.pntd.0004533. eCollection 2016 Mar. PLoS Negl Trop Dis. 2016. PMID: 26991453 Free PMC article.
-
Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates.Curr Genet. 2005 Nov;48(5):277-99. doi: 10.1007/s00294-005-0027-0. Epub 2005 Nov 4. Curr Genet. 2005. PMID: 16215758 Review.
References
-
- Bhat, G.J., Souza, A.E., Feagin, J.E., and Stuart, K. 1992. Transcript-specific developmental regulation of polyadenylation in Trypanosoma brucei mitochondria. Mol. Biochem. Parasitol. 52: 231–240. - PubMed
-
- Blum, B., Bakalara, N., and Simpson, L. 1990. A model for RNA editing in kinetoplastid mitochondria: ‘Guide’ RNA molecules transcribed from maxicircle DNA provide the edited information. Cell 60: 189–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
