Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model
- PMID: 15146234
- PMCID: PMC406534
- DOI: 10.1172/JCI21746
Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model
Abstract
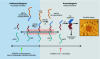
The amyloid beta-peptide (A beta peptide) is assumed to play a crucial and early role in the pathogenesis of Alzheimer disease. Thus, strategies for a pharmacotherapy aim at reducing A beta peptide generation, which proteolytically derives from the amyloid precursor protein (APP). The main targets so far have been beta- and gamma-secretase, the two proteases that cleave APP at the N- and C-terminus of the A beta peptide and are thus directly responsible for A beta peptide generation. A different strategy, namely the activation of alpha-secretase, has barely been investigated for its therapeutic potential. alpha-Secretase cleaves within the A beta peptide domain and thus precludes A beta peptide generation. Now, new results demonstrate that activation of alpha-secretase indeed reduces A beta peptide generation and toxicity in vivo.
Figures
Comment on
-
A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model.J Clin Invest. 2004 May;113(10):1456-64. doi: 10.1172/JCI20864. J Clin Invest. 2004. PMID: 15146243 Free PMC article.
References
-
- Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts myloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. - PubMed
-
- Citron M. beta-Secretase inhibition for the treatment of Alzheimer’s disease - promise and challenge. Trends Pharmacol. Sci. 2004;25:92–97. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

