Induction of B7-1 in podocytes is associated with nephrotic syndrome
- PMID: 15146236
- PMCID: PMC406528
- DOI: 10.1172/JCI20402
Induction of B7-1 in podocytes is associated with nephrotic syndrome
Abstract
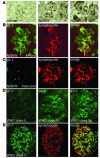
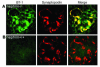
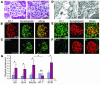
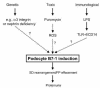
Kidney podocytes and their slit diaphragms form the final barrier to urinary protein loss. This explains why podocyte injury is typically associated with nephrotic syndrome. The present study uncovered an unanticipated novel role for costimulatory molecule B7-1 in podocytes as an inducible modifier of glomerular permselectivity. B7-1 in podocytes was found in genetic, drug-induced, immune-mediated, and bacterial toxin-induced experimental kidney diseases with nephrotic syndrome. The clinical significance of our results is underscored by the observation that podocyte expression of B7-1 correlated with the severity of human lupus nephritis. In vivo, exposure to low-dose LPS rapidly upregulates B7-1 in podocytes of WT and SCID mice, leading to nephrotic-range proteinuria. Mice lacking B7-1 are protected from LPS-induced nephrotic syndrome, suggesting a link between podocyte B7-1 expression and proteinuria. LPS signaling through toll-like receptor-4 reorganized the podocyte actin cytoskeleton in vitro, and activation of B7-1 in cultured podocytes led to reorganization of vital slit diaphragm proteins. In summary, upregulation of B7-1 in podocytes may contribute to the pathogenesis of proteinuria by disrupting the glomerular filter and provides a novel molecular target to tackle proteinuric kidney diseases. Our findings suggest a novel function for B7-1 in danger signaling by nonimmune cells.
Figures



References
-
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat. Genet. 2000;24:333–335. - PubMed
-
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. - PubMed
-
- Kestila M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. - PubMed
-
- Shih NY, et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. - PubMed
-
- Kaplan JM, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 2000;24:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

