Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c
- PMID: 15146238
- PMCID: PMC406532
- DOI: 10.1172/JCI21025
Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c
Abstract
We explored the effects of bile acids on triglyceride (TG) homeostasis using a combination of molecular, cellular, and animal models. Cholic acid (CA) prevents hepatic TG accumulation, VLDL secretion, and elevated serum TG in mouse models of hypertriglyceridemia. At the molecular level, CA decreases hepatic expression of SREBP-1c and its lipogenic target genes. Through the use of mouse mutants for the short heterodimer partner (SHP) and liver X receptor (LXR) alpha and beta, we demonstrate the critical dependence of the reduction of SREBP-1c expression by either natural or synthetic farnesoid X receptor (FXR) agonists on both SHP and LXR alpha and LXR beta. These results suggest that strategies aimed at increasing FXR activity and the repressive effects of SHP should be explored to correct hypertriglyceridemia.
Figures

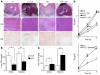

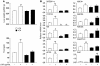
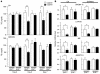

Similar articles
-
Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements.J Biol Chem. 2002 Jan 18;277(3):1705-11. doi: 10.1074/jbc.M105711200. Epub 2001 Nov 2. J Biol Chem. 2002. PMID: 11694526
-
Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver.Am J Physiol Gastrointest Liver Physiol. 2005 Nov;289(5):G798-805. doi: 10.1152/ajpgi.00319.2004. Epub 2005 Jul 7. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 16002565
-
Farnesoid X receptor is essential for normal glucose homeostasis.J Clin Invest. 2006 Apr;116(4):1102-9. doi: 10.1172/JCI25604. Epub 2006 Mar 23. J Clin Invest. 2006. PMID: 16557297 Free PMC article.
-
Triglycerides and gallstone formation.Clin Chim Acta. 2010 Nov 11;411(21-22):1625-31. doi: 10.1016/j.cca.2010.08.003. Epub 2010 Aug 10. Clin Chim Acta. 2010. PMID: 20699090 Review.
-
[Eicosapentaenoic acid].Nihon Rinsho. 2001 Mar;59 Suppl 3:650-6. Nihon Rinsho. 2001. PMID: 11347148 Review. Japanese. No abstract available.
Cited by
-
Role of bile acids in the regulation of the metabolic pathways.World J Diabetes. 2016 Jul 10;7(13):260-70. doi: 10.4239/wjd.v7.i13.260. World J Diabetes. 2016. PMID: 27433295 Free PMC article. Review.
-
Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus.PLoS One. 2011;6(11):e25006. doi: 10.1371/journal.pone.0025006. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110577 Free PMC article.
-
Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways.Nat Med. 2012 Nov;18(11):1665-72. doi: 10.1038/nm.2962. Epub 2012 Oct 28. Nat Med. 2012. PMID: 23104131 Free PMC article.
-
Reply.Hepatology. 2016 May;63(5):1740-1. doi: 10.1002/hep.27961. Epub 2015 Jul 31. Hepatology. 2016. PMID: 26121965 Free PMC article. No abstract available.
-
Cholesteryl ester transfer protein alters liver and plasma triglyceride metabolism through two liver networks in female mice.J Lipid Res. 2016 Aug;57(8):1541-51. doi: 10.1194/jlr.M069013. Epub 2016 Jun 27. J Lipid Res. 2016. PMID: 27354419 Free PMC article.
References
-
- Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am. J. Cardiol. 2000;86:943–949. - PubMed
-
- Ginsberg HN. Hypertriglyceridemia: new insights and new approaches to pharmacologic therapy. Am. J. Cardiol. 2001;87:1174–1180. - PubMed
-
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996;37:907–925. - PubMed
-
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

