Cortical spreading depression activates and upregulates MMP-9
- PMID: 15146242
- PMCID: PMC406541
- DOI: 10.1172/JCI21227
Cortical spreading depression activates and upregulates MMP-9
Abstract
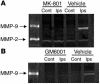
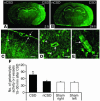
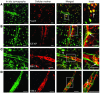
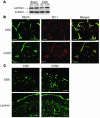
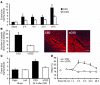
Cortical spreading depression (CSD) is a propagating wave of neuronal and glial depolarization and has been implicated in disorders of neurovascular regulation such as stroke, head trauma, and migraine. In this study, we found that CSD alters blood-brain barrier (BBB) permeability by activating brain MMPs. Beginning at 3-6 hours, MMP-9 levels increased within cortex ipsilateral to the CSD, reaching a maximum at 24 hours and persisting for at least 48 hours. Gelatinolytic activity was detected earliest within the matrix of cortical blood vessels and later within neurons and pia arachnoid (> or =3 hours), particularly within piriform cortex; this activity was suppressed by injection of the metalloprotease inhibitor GM6001 or in vitro by the addition of a zinc chelator (1,10-phenanthroline). At 3-24 hours, immunoreactive laminin, endothelial barrier antigen, and zona occludens-1 diminished in the ipsilateral cortex, suggesting that CSD altered proteins critical to the integrity of the BBB. At 3 hours after CSD, plasma protein leakage and brain edema developed contemporaneously. Albumin leakage was suppressed by the administration of GM6001. Protein leakage was not detected in MMP-9-null mice, implicating the MMP-9 isoform in barrier disruption. We conclude that intense neuronal and glial depolarization initiates a cascade that disrupts the BBB via an MMP-9-dependent mechanism.
Figures



References
-
- Sugaya E, Takato M, Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J. Neurophysiol. 1975;38:822–841. - PubMed
-
- Kager H, Wadman WJ, Somjen GG. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. J. Neurophysiol. 2000;84:495–512. - PubMed
-
- Leao AAP. Pial circulation and spreading depression activity in cerebral cortex. J. Neurophysiol. 1944;7:391–396.
-
- Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol. Scand. 2000;102:275–283. - PubMed
-
- Martins-Ferreira H, Nedergaard M, Nicholson C. Perspectives on spreading depression. Brain Res. Brain Res. Rev. 2000;32:215–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

